Fall 2006 - College of Dental Medicine - Columbia University
Fall 2006 - College of Dental Medicine - Columbia University
Fall 2006 - College of Dental Medicine - Columbia University
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
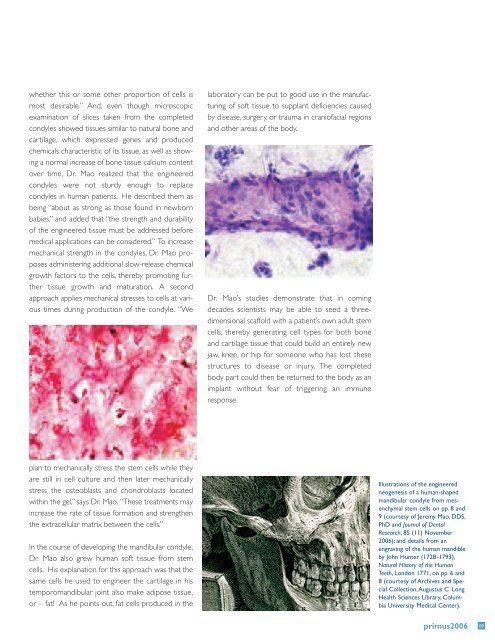
whether this or some other proportion <strong>of</strong> cells ismost desirable.” And, even though microscopicexamination <strong>of</strong> slices taken from the completedcondyles showed tissues similar to natural bone andcartilage, which expressed genes and producedchemicals characteristic <strong>of</strong> its tissue, as well as showinga normal increase <strong>of</strong> bone tissue calcium contentover time, Dr. Mao realized that the engineeredcondyles were not sturdy enough to replacecondyles in human patients. He described them asbeing “about as strong as those found in newbornbabies,” and added that “the strength and durability<strong>of</strong> the engineered tissue must be addressed beforemedical applications can be considered.” To increasemechanical strength in the condyles, Dr. Mao proposesadministering additional slow-release chemicalgrowth factors to the cells, thereby promoting furthertissue growth and maturation. A secondapproach applies mechanical stresses to cells at varioustimes during production <strong>of</strong> the condyle. “Welaboratory can be put to good use in the manufacturing<strong>of</strong> s<strong>of</strong>t tissue to supplant deficiencies causedby disease, surgery, or trauma in crani<strong>of</strong>acial regionsand other areas <strong>of</strong> the body.Dr. Mao’s studies demonstrate that in comingdecades scientists may be able to seed a threedimensionalscaffold with a patient’s own adult stemcells, thereby generating cell types for both boneand cartilage tissue that could build an entirely newjaw, knee, or hip for someone who has lost thesestructures to disease or injury. The completedbody part could then be returned to the body as animplant without fear <strong>of</strong> triggering an immuneresponse.plan to mechanically stress the stem cells while theyare still in cell culture and then later mechanicallystress the osteoblasts and chondroblasts locatedwithin the gel,” says Dr. Mao. “These treatments mayincrease the rate <strong>of</strong> tissue formation and strengthenthe extracellular matrix between the cells.”In the course <strong>of</strong> developing the mandibular condyle,Dr. Mao also grew human s<strong>of</strong>t tissue from stemcells. His explanation for this approach was that thesame cells he used to engineer the cartilage in histemporomandibular joint also make adipose tissue,or – fat! As he points out, fat cells produced in theIllustrations <strong>of</strong> the engineeredneogenesis <strong>of</strong> a human-shapedmandibular condyle from mesenchymalstem cells on pp. 8 and9 (courtesy <strong>of</strong> Jeremy Mao, DDS,PhD and Journal <strong>of</strong> <strong>Dental</strong>Research, 85 (11) November<strong>2006</strong>); and details from anengraving <strong>of</strong> the human mandibleby John Hunter (1728-1793),Natural History <strong>of</strong> the HumanTeeth, London 1771, on pp. 6 and8 (courtesy <strong>of</strong> Archives and SpecialCollection,Augustus C. LongHealth Sciences Library, <strong>Columbia</strong><strong>University</strong> Medical Center).primus<strong>2006</strong> 09