Determination of inorganic anions in red algae (Eucheuma cottonii ...
Determination of inorganic anions in red algae (Eucheuma cottonii ...
Determination of inorganic anions in red algae (Eucheuma cottonii ...
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
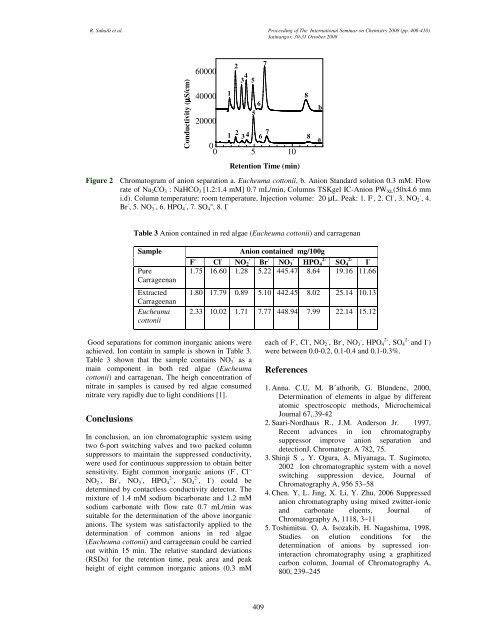
R. Suhaili et al. Proceed<strong>in</strong>g <strong>of</strong> The International Sem<strong>in</strong>ar on Chemistry 2008 (pp. 406-410)Jat<strong>in</strong>angor, 30-31 October 2008Conductivity (µS/cm)600004000020000123 4 5651 2 3 4 6 7 800 5 10Figure 2 Chromatogram <strong>of</strong> anion separation a. <strong>Eucheuma</strong> <strong>cottonii</strong>, b. Anion Standard solution 0.3 mM. Flowrate <strong>of</strong> Na 2 CO 3 : NaHCO 3 [1.2:1.4 mM] 0.7 mL/m<strong>in</strong>, Columns TSKgel IC-Anion PW XL (50x4.6 mmi.d). Column temperature: room temperature, Injection volume: 20 µL. Peak: 1. F - , 2. Cl - , 3. NO 2 - , 4.Br - , 5. NO 3 - , 6. HPO 4 - , 7. SO 4 = , 8. I -7Retention Time (m<strong>in</strong>)8baTable 3 Anion conta<strong>in</strong>ed <strong>in</strong> <strong>red</strong> <strong>algae</strong> (<strong>Eucheuma</strong> <strong>cottonii</strong>) and carragenanSamplePureCarrageenanExtractedCarrageenan<strong>Eucheuma</strong><strong>cottonii</strong>Anion conta<strong>in</strong>ed mg/100gF - Cl - -NO 2 Br - -NO 32-HPO 42-SO 4 I -1.75 16.60 1.28 5.22 445.47 8.64 19.16 11.661.80 17.79 0.89 5.10 442.45 8.02 25.14 10.132.33 10.02 1.71 7.77 448.94 7.99 22.14 15.12Good separations for common <strong><strong>in</strong>organic</strong> <strong>anions</strong> wereachieved. Ion conta<strong>in</strong> <strong>in</strong> sample is shown <strong>in</strong> Table 3.-Table 3 shown that the sample conta<strong>in</strong>s NO 3 as ama<strong>in</strong> component <strong>in</strong> both <strong>red</strong> <strong>algae</strong> (<strong>Eucheuma</strong><strong>cottonii</strong>) and carragenan. The heigh concentration <strong>of</strong>nitrate <strong>in</strong> samples is caused by <strong>red</strong> <strong>algae</strong> consumednitrate very rapidly due to light conditions [1].ConclusionsIn conclusion, an ion chromatographic system us<strong>in</strong>gtwo 6-port switch<strong>in</strong>g valves and two packed columnsuppressors to ma<strong>in</strong>ta<strong>in</strong> the suppressed conductivity,were used for cont<strong>in</strong>uous suppression to obta<strong>in</strong> bettersensitivity. Eight common <strong><strong>in</strong>organic</strong> <strong>anions</strong> (F - , Cl -,NO 2 - , Br - , NO 3 - , HPO 4 2- , SO 4 2- , I - ) could bedeterm<strong>in</strong>ed by contactless conductivity detector. Themixture <strong>of</strong> 1.4 mM sodium bicarbonate and 1.2 mMsodium carbonate with flow rate 0.7 mL/m<strong>in</strong> wassuitable for the determ<strong>in</strong>ation <strong>of</strong> the above <strong><strong>in</strong>organic</strong><strong>anions</strong>. The system was satisfactorily applied to thedeterm<strong>in</strong>ation <strong>of</strong> common <strong>anions</strong> <strong>in</strong> <strong>red</strong> <strong>algae</strong>(<strong>Eucheuma</strong> <strong>cottonii</strong>) and carrageenan could be carriedout with<strong>in</strong> 15 m<strong>in</strong>. The relative standard deviations(RSDs) for the retention time, peak area and peakheight <strong>of</strong> eight common <strong><strong>in</strong>organic</strong> <strong>anions</strong> (0.3 mMeach <strong>of</strong> F - , Cl - , NO 2 - , Br - , NO 3 - , HPO 4 2- , SO 42-and I - )were between 0.0-0.2, 0.1-0.4 and 0.1-0.3%.References1. Anna. C.U, M. B´athorib, G. Blundenc, 2000,<strong>Determ<strong>in</strong>ation</strong> <strong>of</strong> elements <strong>in</strong> <strong>algae</strong> by differentatomic spectroscopic methods, MicrochemicalJournal 67,.39-422. Saari-Nordhaus R., J.M. Anderson Jr. 1997,Recent advances <strong>in</strong> ion chromatographysuppressor improve anion separation anddetectionJ. Chromatogr. A 782, 75.3. Sh<strong>in</strong>ji S ,, Y. Ogura, A. Miyanaga, T. Sugimoto,2002 Ion chromatographic system with a novelswitch<strong>in</strong>g suppression device, Journal <strong>of</strong>Chromatography A, 956 53–584. Chen. Y, L. J<strong>in</strong>g, X. Li, Y. Zhu, 2006 Suppressedanion chromatography us<strong>in</strong>g mixed zwitter-ionicand carbonate eluents, Journal <strong>of</strong>Chromatography A, 1118, 3–115. Toshimitsu. O, A. Isozakib, H. Nagashima, 1998,Studies on elution conditions for thedeterm<strong>in</strong>ation <strong>of</strong> <strong>anions</strong> by supressed ion<strong>in</strong>teractionchromatography us<strong>in</strong>g a graphitizedcarbon column, Journal <strong>of</strong> Chromatography A,800, 239–245409