01-29-13 Stoichiometry Introduction Notes ACh.pdf - Whitnall High ...
01-29-13 Stoichiometry Introduction Notes ACh.pdf - Whitnall High ...
01-29-13 Stoichiometry Introduction Notes ACh.pdf - Whitnall High ...
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
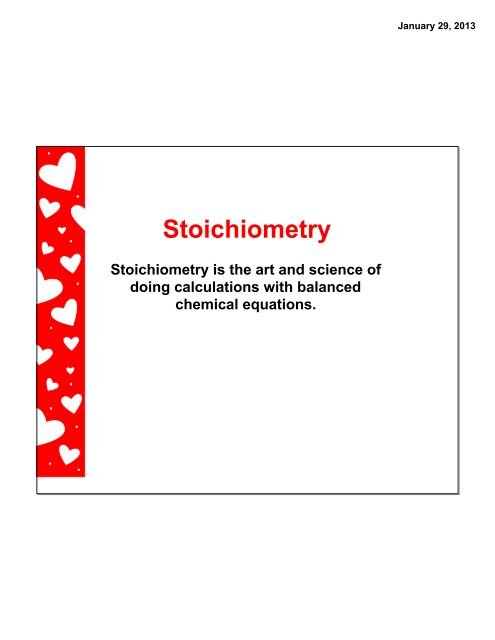
January <strong>29</strong>, 2<strong>01</strong>3<strong>Stoichiometry</strong><strong>Stoichiometry</strong> is the art and science ofdoing calculations with balancedchemical equations.
January <strong>29</strong>, 2<strong>01</strong>3<strong>Stoichiometry</strong> comesfrom two Greek words.Stoicheion meaning elementandMetron meaning measure
January <strong>29</strong>, 2<strong>01</strong>3<strong>Stoichiometry</strong>Process used when you are given anamount of one kind of substance andyou need to know the amount of anotherkind of substance. It all starts with acorrectly written and balanced chemicalequation!
January <strong>29</strong>, 2<strong>01</strong>3Student will be able to:1. describe the process of stoichiometry and identify whenit needs to be used.2. determine mole ratios for each reactant and product in achemical reaction.
January <strong>29</strong>, 2<strong>01</strong>3First the hamburger analogyA recipe for a bacon double cheeseburger is:· 1 hamburger bun· 2 hamburger patties· 3 slices of cheese· 4 strips of baconBased upon this recipe:If I have five bacon double cheeseburgers:How many hamburger buns did I use?How many hamburger patties did I use?How many slices of cheese did I use?How many strips of bacon did I use?
January <strong>29</strong>, 2<strong>01</strong>3Chemistry problemA recipe for sodium chloride:2Na + Cl 22NaClCoefficients in a balanced chemical equation have 2 meanings.microscopic level:2 atoms Na react with 1 molecule Cl 2to produce2 formula units NaCl.molar level:2 mol Na react with 1 mol Cl 2to produce 2 mol NaCl.
January <strong>29</strong>, 2<strong>01</strong>3Chemistry problemA recipe for sodium chloride:2Na + Cl 22NaClIf I made 4 moles of sodium chloride:How many moles of sodium did I use?How many moles of chlorine gas did I use?
January <strong>29</strong>, 2<strong>01</strong>3Back to the hamburger analogyA recipe for a bacon double cheeseburger is:· 1 hamburger bun· 2 hamburger patties· 3 slices of cheese· 4 strips of baconBased on this recipe:How many bacon double cheeseburgers can youmake if you start with:1 mole bun, 2 mole patties, 3 mole slices ofcheese, 4 mole strips of bacon?10 buns, 20 patties, 3 slices of cheese,40 strips of bacon?
January <strong>29</strong>, 2<strong>01</strong>3Chemistry problemA recipe for sodium chloride:2Na + Cl 22NaClIf I had 6 moles of sodium and 12 moles of chlorine:How many moles of sodium chloride can I make?How many moles of chlorine will be left over?
January <strong>29</strong>, 2<strong>01</strong>3Back to the hamburger analogyA recipe for a bacon double cheeseburger is:· 1 hamburger bun· 2 hamburger patties· 3 slices of cheese· 4 strips of baconIf you had fixings for 100 bacon double cheeseburgers,but when you were cooking you ruined 10 of them.What percentage of the bacon double cheeseburgersdid you actually make?
January <strong>29</strong>, 2<strong>01</strong>3Chemistry problemA recipe for sodium chloride:2Na + Cl 22NaClIn the lab you used 2 moles of sodium and 1 mole ofchlorine gas, after careful analysis you determine thatyou have produced 1 mole of sodium chloride. Whatwas your percent yield?
The math and theconcepts will remainthe same, the onlydifference will be in therecipe. In chemistryyour recipe will be abalanced chemicalequation.January <strong>29</strong>, 2<strong>01</strong>3
January <strong>29</strong>, 2<strong>01</strong>3Coefficients in a balanced chemicalequation have 2 levels of interpretation.4NH 3+ 5O 24NO + 6H 2Omicroscopic level (4 molecules of NH 3)molar level (4 moles of NH 3)
January <strong>29</strong>, 2<strong>01</strong>3