Electrochemical Oxidation of Mn - unist
Electrochemical Oxidation of Mn - unist
Electrochemical Oxidation of Mn - unist
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
E268<br />
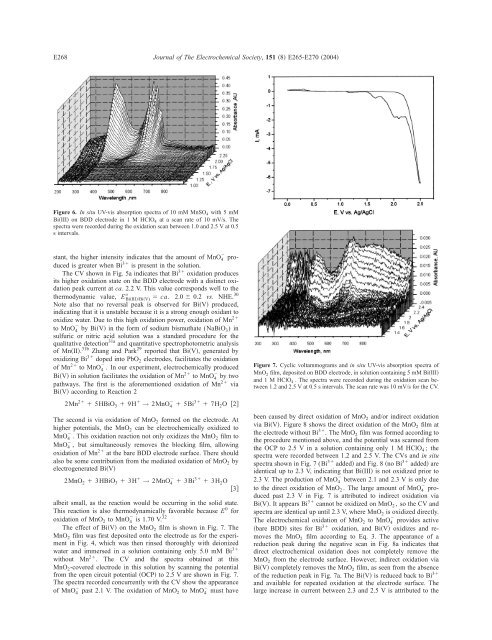
Figure 6. In situ UV-vis absorption spectra <strong>of</strong> 10 mM <strong>Mn</strong>SO 4 with5mM<br />
Bi�III� on BDD electrode in 1MHClO 4 at a scan rate <strong>of</strong> 10 mV/s. The<br />
spectra were recorded during the oxidation scan between 1.0 and 2.5 V at 0.5<br />
s intervals.<br />
stant, the higher intensity indicates that the amount <strong>of</strong> <strong>Mn</strong>O 4 � produced<br />
is greater when Bi 3� is present in the solution.<br />
The CV shown in Fig. 5a indicates that Bi 3� oxidation produces<br />
its higher oxidation state on the BDD electrode with a distinct oxi-<br />
dation peak current at ca. 2.2 V. This value corresponds well to the<br />
o<br />
thermodynamic value, EBi�III�/Bi�V) � ca. 2.0 � 0.2 vs. NHE. 30<br />
Note also that no reversal peak is observed for Bi�V� produced,<br />
indicating that it is unstable because it is a strong enough oxidant to<br />
oxidize water. Due to this high oxidation power, oxidation <strong>of</strong> <strong>Mn</strong> 2�<br />
to <strong>Mn</strong>O 4 � by Bi�V� in the form <strong>of</strong> sodium bismuthate (NaBiO3) in<br />
sulfuric or nitric acid solution was a standard procedure for the<br />
qualitative detection 31a and quantitative spectrophotometric analysis<br />
<strong>of</strong> <strong>Mn</strong>�II�. 31b Zhang and Park 29 reported that Bi�V�, generated by<br />
oxidizing Bi 3� doped into PbO 2 electrodes, facilitates the oxidation<br />
<strong>of</strong> <strong>Mn</strong> 2� to <strong>Mn</strong>O 4 � . In our experiment, electrochemically produced<br />
Bi�V� in solution facilitates the oxidation <strong>of</strong> <strong>Mn</strong> 2� to <strong>Mn</strong>O 4 � by two<br />
pathways. The first is the aforementioned oxidation <strong>of</strong> <strong>Mn</strong> 2� via<br />
Bi�V� according to Reaction 2<br />
2<strong>Mn</strong> 2� � 5HBiO 3 � 9H � → 2<strong>Mn</strong>O 4 � � 5Bi 3� � 7H2O �2�<br />
The second is via oxidation <strong>of</strong> <strong>Mn</strong>O 2 formed on the electrode. At<br />
higher potentials, the <strong>Mn</strong>O 2 can be electrochemically oxidized to<br />
<strong>Mn</strong>O 4 � . This oxidation reaction not only oxidizes the <strong>Mn</strong>O2 film to<br />
<strong>Mn</strong>O 4 � , but simultaneously removes the blocking film, allowing<br />
oxidation <strong>of</strong> <strong>Mn</strong> 2� at the bare BDD electrode surface. There should<br />
also be some contribution from the mediated oxidation <strong>of</strong> <strong>Mn</strong>O 2 by<br />
electrogenerated Bi�V�<br />
2<strong>Mn</strong>O2 � 3HBiO3 � 3H� � 3� → 2<strong>Mn</strong>O4 � 3Bi � 3H2O<br />
�3�<br />
albeit small, as the reaction would be occurring in the solid state.<br />
This reaction is also thermodynamically favorable because E 0 for<br />
oxidation <strong>of</strong> <strong>Mn</strong>O 2 to <strong>Mn</strong>O 4 � is 1.70 V. 32<br />
The effect <strong>of</strong> Bi�V� on the <strong>Mn</strong>O 2 film is shown in Fig. 7. The<br />
<strong>Mn</strong>O 2 film was first deposited onto the electrode as for the experiment<br />
in Fig. 4, which was then rinsed thoroughly with deionized<br />
water and immersed in a solution containing only 5.0 mM Bi 3�<br />
without <strong>Mn</strong> 2� . The CV and the spectra obtained at this<br />
<strong>Mn</strong>O 2-covered electrode in this solution by scanning the potential<br />
from the open circuit potential �OCP� to 2.5 V are shown in Fig. 7.<br />
The spectra recorded concurrently with the CV show the appearance<br />
<strong>of</strong> <strong>Mn</strong>O 4 � past 2.1 V. The oxidation <strong>of</strong> <strong>Mn</strong>O2 to <strong>Mn</strong>O 4 � must have<br />
Journal <strong>of</strong> The <strong>Electrochemical</strong> Society, 151 �8� E265-E270 �2004�<br />
Figure 7. Cyclic voltammograms and in situ UV-vis absorption spectra <strong>of</strong><br />
<strong>Mn</strong>O 2 film, deposited on BDD electrode, in solution containing 5 mM Bi�III�<br />
and1MHClO 4 . The spectra were recorded during the oxidation scan between<br />
1.2 and 2.5 V at 0.5 s intervals. The scan rate was 10 mV/s for the CV.<br />
been caused by direct oxidation <strong>of</strong> <strong>Mn</strong>O 2 and/or indirect oxidation<br />
via Bi�V�. Figure 8 shows the direct oxidation <strong>of</strong> the <strong>Mn</strong>O 2 film at<br />
the electrode without Bi 3� . The <strong>Mn</strong>O 2 film was formed according to<br />
the procedure mentioned above, and the potential was scanned from<br />
the OCP to 2.5 V in a solution containing only 1 M HClO 4 ; the<br />
spectra were recorded between 1.2 and 2.5 V. The CVs and in situ<br />
spectra shown in Fig. 7 (Bi 3� added� and Fig. 8 �no Bi 3� added� are<br />
identical up to 2.3 V, indicating that Bi�III� is not oxidized prior to<br />
2.3 V. The production <strong>of</strong> <strong>Mn</strong>O 4 � between 2.1 and 2.3 V is only due<br />
to the direct oxidation <strong>of</strong> <strong>Mn</strong>O 2 . The large amount <strong>of</strong> <strong>Mn</strong>O 4 � produced<br />
past 2.3 V in Fig. 7 is attributed to indirect oxidation via<br />
Bi�V�. It appears Bi 3� cannot be oxidized on <strong>Mn</strong>O 2 , so the CV and<br />
spectra are identical up until 2.3 V, where <strong>Mn</strong>O 2 is oxidized directly.<br />
The electrochemical oxidation <strong>of</strong> <strong>Mn</strong>O 2 to <strong>Mn</strong>O 4 � provides active<br />
�bare BDD� sites for Bi 3� oxidation, and Bi�V� oxidizes and removes<br />
the <strong>Mn</strong>O 2 film according to Eq. 3. The appearance <strong>of</strong> a<br />
reduction peak during the negative scan in Fig. 8a indicates that<br />
direct electrochemical oxidation does not completely remove the<br />
<strong>Mn</strong>O 2 from the electrode surface. However, indirect oxidation via<br />
Bi�V� completely removes the <strong>Mn</strong>O 2 film, as seen from the absence<br />
<strong>of</strong> the reduction peak in Fig. 7a. The Bi�V� is reduced back to Bi 3�<br />
and available for repeated oxidation at the electrode surface. The<br />
large increase in current between 2.3 and 2.5 V is attributed to the