Characterizing Protein/Ligand Binding by DSC - LNBio
Characterizing Protein/Ligand Binding by DSC - LNBio
Characterizing Protein/Ligand Binding by DSC - LNBio
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
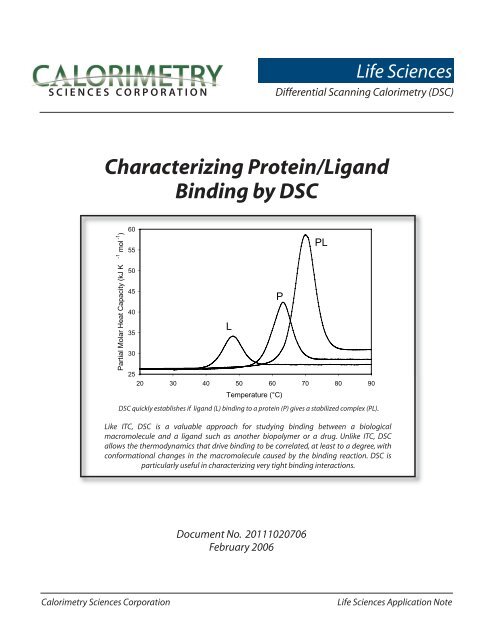
SCIENCES CORPORATIONLife SciencesDifferential Scanning Calorimetry (<strong>DSC</strong>)<strong>Characterizing</strong> <strong>Protein</strong>/<strong>Ligand</strong><strong>Binding</strong> <strong>by</strong> <strong>DSC</strong>Partial Molar Heat Capacity (kJ K -1 mol -1 )60555045403530L2520 30 40 50 60 70 80 90Temperature (°C)<strong>DSC</strong> quickly establishes if ligand (L) binding to a protein (P) gives a stabilized complex (PL).Like ITC, <strong>DSC</strong> is a valuable approach for studying binding between a biologicalmacromolecule and a ligand such as another biopolymer or a drug. Unlike ITC, <strong>DSC</strong>allows the thermodynamics that drive binding to be correlated, at least to a degree, withconformational changes in the macromolecule caused <strong>by</strong> the binding reaction. <strong>DSC</strong> isparticularly useful in characterizing very tight binding interactions.PPLDocument No. 20111020706February 2006Calorimetry Sciences CorporationLife Sciences Application Note
All proteins are capable of recognizing andbinding specific molecules such as other proteins,cofactors, prosthetic groups or drugs. Effortsto understand the mechanisms controlling selectivebinding were initially prompted <strong>by</strong> the realization thatrecognition and binding are universal features of allbiochemical processes. These efforts have intensifiedwith the awareness that knowledge-based drug designrequires not only high-quality structural data on both theprotein and the drug candidate, but also a quantitativeunderstanding of the thermodynamics driving binding.A ligand will bind to a protein (or other macromolecule)only if the resulting complex is more stable than theoriginal, non-liganded protein. <strong>Binding</strong> can occur tothe native, folded protein (stabilizing the native state),or it can bind preferentially to the denatured protein, inwhich case the ligand will destabilize the native protein.In either case, binding triggers changes in intramolecularand intermolecular interactions, and in the dynamics ofboth the protein and the ligand. Since the degree ofstabilization or destabilization of the native proteindepends on the magnitude of the binding energy,comparison of the stability of the complex with thestability of the ligand-free protein allows the bindingenergy to be estimated.Differential scanning calorimetry (<strong>DSC</strong>) is particularlysuited to studying the thermodynamics controllingconformational transitions in macromolecules such asproteins. As explained in CSC’s overview note entitled‘Life Science Applications of <strong>DSC</strong>’, <strong>DSC</strong> is generally usedto measure the partial molar heat capacity of a proteinover a temperature range of approximately 80 o C. If aligand binds preferentially to the native state of theprotein, the temperature at which the protein-ligandcomplex denatures will be higher compared to thetemperature at which the free protein unfolds. <strong>DSC</strong> thusprovides a direct measure of whether ligand bindingto a protein is stabilizing or destabilizing, and so cancomplement studies of binding equilibria obtained <strong>by</strong>isothermal titration calorimetry (ITC). For a discussionof the utility of ITC in binding studies, please see CSC’sapplication note entitled ‘<strong>Characterizing</strong> protein/ligandbinding <strong>by</strong> ITC’.Assessing ligand binding <strong>by</strong> <strong>DSC</strong><strong>Protein</strong>s are large and flexible, and constantlysample the continuum of conformational states frompartially folded to fully native. The native state itself is notone conformation, but a rapidly-changing ensemble ofclosely related structures (Ringe and Petsko, 1985; Palmer1997). The binding between a flexible protein and a smallligand therefore produces a complex energy profileinvolving many favorable and unfavorable contributions.The thermodynamic assessment of a binding event isfurther complicated <strong>by</strong> the fact that ligands such as drugmolecules often contain hydrophobic moieties whichinteract with hydrophobic patches on the surface (or inthe binding crevice) of the protein. Water is more orderedadjacent to hydrophobic surfaces (Shinoda, 1977); whenthe protein and ligand bind and hydrophobic surfacesinteract, bound water at these surfaces is transferredinto the bulk solvent. Since the free energy of the entiresystem must decrease if binding is to occur, these solventeffects must be accounted for in addition to the energychanges resulting from direct noncovalent interactionsbetween the protein and ligand (Jelesarov and Bosshard,1999).ITC is the most direct approach for assessing bindinginteractions: several rapid ITC experiments can providea direct determination of the binding constant, thestoichiometry of binding, the enthalpy and entropy ofthe reaction and the change in heat capacity due tobinding. However, as noted in the CSC ITC applicationnote on binding studies, the most accurate bindingconstant measurements are obtained for reactionswith medium to tight binding. Manipulation of theexperimental conditions allows very weak and verytight association constants to be determined <strong>by</strong> ITC,but an independent assessment of K ais advantageousin these cases. Since <strong>DSC</strong> compares the extent to whichthe ligand/protein complex is stabilized towards thermaldenaturation compared to the free protein and ligand,<strong>DSC</strong> allows determination of binding energies for verytightly associated complexes, or complexes whichequilibrate very slowly (minutes to hours) and thusare not compatible with the ITC timeframe (seconds)(Holdgate and Ward, 2005). <strong>DSC</strong> also allows the numberof intermediate states in the binding pathway to bedetermined, whereas ITC ideally requires that the bindingprocess be two-state. In addition, <strong>by</strong> conducting several<strong>DSC</strong> binding experiments at various concentrations ofreactants, the population of free and bound speciescan be calculated as a function of temperature andconcentration (Holdgate and Ward, 2002).Calorimetry Sciences CorporationLife Sciences Application Note
Partial Molar Heat Capacity (kJ K -1 mol -1 )60555045403530L2520 30 40 50 60 70 80 90Fig. 1. Simulated <strong>DSC</strong> data showing the molar heat capacity offree ligand (L), free protein (P) and the protein-ligand complex(PL), where the complex is a tightly-associating system.If a ligand (L) binds to the native conformation ofa protein (P) with high affinity, the resulting protein/ligand complex (PL) will have a higher thermal stabilitythan either of the two free components (Jelesarov andBosshard, 1999). Figure 1 shows an idealized examplewhere the stabilized complex has a significantly highermelting temperature than the protein or ligand alone.The symmetry of this PL peak suggests that complexdissociation and thermal denaturation are tightlycoupled. In practice, the shape of the protein/ligandendotherm can be quite complex due to the presence ofintermediates and/or denatured protein, the sensitivityof the complex to pH and ionic strength, or to theligand preferentially binding to and stabilizing onlypart of the protein (e.g., one domain) (Brandts and Lin,1990; Jelesarov and Bosshard, 1999; Luque et al., 2002).For many applications, only an approximation of theT mof the complex (the temperature at which half theprotein/ligand complex molecules are folded and halfare unfolded) vs. the T mof the free protein is required;this information can generally be obtained <strong>by</strong> visualinspection of the endotherms. However, since the freeenergy of complex formation results from a delicatebalance between large favorable and unfavorableentropic and enthalpic contributions, precautions mustbe taken not to over-interpret the data. For example, alarge observed T mshift is not necessarily an indication ofhigh affinity binding, since a range of different affinities,with different entropic and enthalpic contributions,could result in the same T m.PTemperature (°C)PLIf determination of the free energy of complexformation is required and the endotherm has a simpleshape (indicative of a two-state process), the free energychange due to binding is calculated <strong>by</strong> subtracting thefree energy change of unfolding of the complex (∆G comp)from the free energy change of unfolding of the protein(∆G prot) (i.e., ∆G = ∆G prot– ∆G comp). If the endothermis complex, indicative of multiple folding states, theendotherm must be deconvoluted to extract the freeenergy contributions of each state. <strong>DSC</strong> and ITC thusallow a binding reaction to be studied from two differentangles. The power of ITC is that it gives accurate values of∆H, and allows the determination of ∆C pif heat capacityis independent of temperature and the interactingmolecules form a two-state complex. The versatility of<strong>DSC</strong> means that although measurements may producea complex endotherm, following deconvolution the dataallow the enthalpy and heat capacity measured <strong>by</strong> ITCto be correlated, at least to a degree, to conformationalchanges occurring upon binding (Jelesarov andBosshard, 1999). The deconvolution process and itsapplication to the analysis of complex binding systemsare described in Jelesarov and Bosshard (1999), Cooperet al. (2001), and references therein.SummaryWhen combined with structural and ITC data, <strong>DSC</strong>provides an independent approach to elucidating thefree energy changes accompanying the formation ofprotein/ligand complexes, which in turn can shed lighton the mechanism of the association reaction. Usedtogether, ITC and <strong>DSC</strong> complement and augment eachother and provide the most direct, comprehensiveapproach for monitoring and interpreting bindinginteractions involving biological macromolecules.Calorimetry Sciences CorporationLife Sciences Application Note
References:(Preference has been given to current references. Citation doesnot imply that a paper is necessarily the original reference to astudy.)Brandts, J. F. and L-N Lin. (1990) Study of strong to ultratightprotein interactions using differential scanningcalorimetry. Biochemistry 29, 6927-6940.Cooper, A., M. A. Nutley and A. Wadood. (2001) Differentialscanning calorimetry. p. 287-318. In S. E. Hardingand B. Z. Chowdry (Eds.) <strong>Protein</strong>-<strong>Ligand</strong> Interactions:hydrodynamics and calorimetry. Oxford University Press,Oxford.Holdgate, G. A. and W. H. J. Ward. (2005) Measurements ofbinding thermodynamics in drug discovery. Drug Discov.Today 10, 1543-1550.Jelesarov, I. and H. R. Bosshard. (1999) Isothermal titrationcalorimetry and differential scanning calorimetry ascomplementary tools to investigate the energetics ofbiomolecular recognition. J. Mol. Recognit. 12, 3-18.Luque, I., S. A. Leavitt and E. Freire. (2002) The linkage betweenprotein folding and functional cooperativity: two sides ofthe same coin? Annu. Rev. Biophys. Biomol. Struct. 31, 235-256.Palmer, A. G. (1997) Probing molecular motion <strong>by</strong> NMR. Curr.Opin. Struct. Biol. 7, 732-737.Ringe, D. and G. A. Petsko. (1985) Mapping protein dynamics <strong>by</strong>X-ray diffraction. Prog. Biophys. Mol. Biol. 45, 197-235.Shinoda, K. (1977) ‘Iceberg’ formation and solubility. J. Phys.Chem. 81, 8069-8072.SCIENCES CORPORATION890 West 410 North, Suite ALindon, Utah 84042Phone: 801.763.1500 Fax: 801.763.1414Web: www.calorimetrysciences.comE-mail: info@calorimetrysciences.comCalorimetry Sciences CorporationLife Sciences Application Note