Cheat Sheet for Exam #1 on Thermodynamics and ... - Chemistry
Cheat Sheet for Exam #1 on Thermodynamics and ... - Chemistry
Cheat Sheet for Exam #1 on Thermodynamics and ... - Chemistry
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
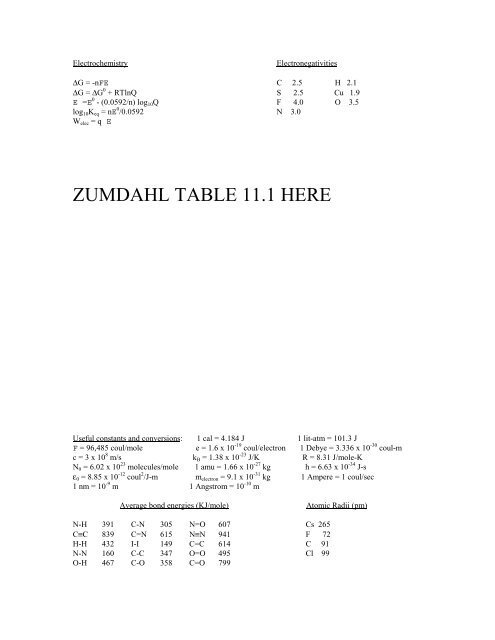
ElectrochemistryElectr<strong>on</strong>egativities∆G = -nFE C 2.5 H 2.1∆G = ∆G 0 + RTlnQ S 2.5 Cu 1.9E =E 0 - (0.0592/n) log 10 Q F 4.0 O 3.5log 10 K eq = nE 0 /0.0592 N 3.0W elec = q EZUMDAHL TABLE 11.1 HEREUseful c<strong>on</strong>stants <strong>and</strong> c<strong>on</strong>versi<strong>on</strong>s: 1 cal = 4.184 J 1 lit-atm = 101.3 JF = 96,485 coul/mole e = 1.6 x 10 -19 coul/electr<strong>on</strong> 1 Debye = 3.336 x 10 -30 coul-mc = 3 x 10 8 m/s k B = 1.38 x 10 -23 J/K R = 8.31 J/mole-KN 0 = 6.02 x 10 23 molecules/mole 1 amu = 1.66 x 10 -27 kg h = 6.63 x 10 -34 J-sε 0 = 8.85 x 10 -12 coul 2 /J-m m electr<strong>on</strong> = 9.1 x 10 -31 kg 1 Ampere = 1 coul/sec1 nm = 10 -9 m 1 Angstrom = 10 -10 mAverage b<strong>on</strong>d energies (KJ/mole)Atomic Radii (pm)N-H 391 C-N 305 N=O 607 Cs 265C≡C 839 C=N 615 N≡N 941 F 72H-H 432 I-I 149 C=C 614 C 91N-N 160 C-C 347 O=O 495 Cl 99O-H 467 C-O 358 C=O 799