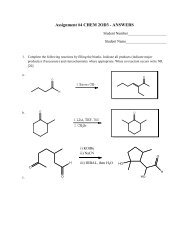
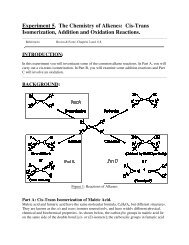
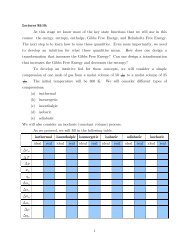
3. Discussion of the Hartree-Fock Equations
3. Discussion of the Hartree-Fock Equations
3. Discussion of the Hartree-Fock Equations
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
where <strong>the</strong> notation indicates than an electron in an occupied orbital, ψ ( )ir is removed from<strong>the</strong> system, becoming a “free” (unbound) electron. Neglecting <strong>the</strong> subsequent relaxation <strong>of</strong><strong>the</strong> remaining occupied orbitals in <strong>the</strong> cation, we can approximate <strong>the</strong> ionization <strong>of</strong> <strong>the</strong>neutral system to form <strong>the</strong> excited state <strong>of</strong> <strong>the</strong> cation byfreeIi ≈ Ei −EHFN N N⎛N N N⎞1 1≈ ∑hk +2∑∑( Jkl −Kkl ) − ⎜∑hk +2∑∑( Jkl −Kkl)⎜⎝⎠⎟k= 1 k= 1 l= 1 k= 1 k= 1 l=1k≠i k≠i l≠i⎛⎞ NN1 1= hi 2 ( Jki Kki) 2 ( Jil Kil) ( Jii Kii)− − ∑ − − ∑ − − − ⎜k= 1 l=1⎜⎝k≠i l≠i⎠⎟⎛⎞ N=− hi ( Jki Kki)+ ∑ − −0⎜k=1⎜⎝ k≠i⎠⎟=−ε i(<strong>3.</strong>15)The fact that <strong>the</strong> orbital energies in <strong>Hartree</strong>-<strong>Fock</strong> <strong>the</strong>ory approximate <strong>the</strong> negative <strong>of</strong> <strong>the</strong>ionization potentials is called Koopmans’ <strong>the</strong>orem. We can approximate <strong>the</strong> “way” <strong>the</strong>electron is removed from <strong>the</strong> molecule by noting that <strong>the</strong> electron has been removed from<strong>the</strong> orbital ψ ( )iz .Particularly important for chemical purposes is <strong>the</strong> highest occupied molecular orbital(HOMO), because I HOMO=− ε HOMOis <strong>the</strong> ground state ionization potential: <strong>the</strong> minimumamount <strong>of</strong> energy it takes to remove an electron from a molecule. Molecules with largehighest-occupied orbital energies (small ionization potentials) are good electrondonors/nucleophiles/Lewis bases/reducing agents. The shape <strong>of</strong> <strong>the</strong> highest-occupied orbitalcontrols <strong>the</strong> regioselectivity <strong>of</strong> <strong>the</strong> nucleophile: if ψ ( ) 2 HOMOr ≈ 0 at a point, <strong>the</strong>n weobserve that <strong>the</strong> it is difficult to remove an electron from <strong>the</strong> molecule at this point becausemost <strong>of</strong> <strong>the</strong> electron density at this point is associated with electrons in orbitals that aremore tightly bound, ε i< ε HOMO. For example, <strong>the</strong> electrophilic attack <strong>of</strong> borane in <strong>the</strong>hydroboration reaction <strong>of</strong> 1,1-diphenyl-2-ethyl-1-butene occurs not on <strong>the</strong> negatively chargedaromatic rings, but at <strong>the</strong> double bond.6