Expt. 2
Expt. 2
Expt. 2
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
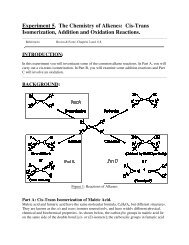
Experiment 2. Separation of Liquids by Distillation.References:Eaton, Laboratory Investigations in Organic ChemistryINTRODUCTION:Experiment 2 illustrates a technique used for the separation of organic liquids – distillation. Itwill also give you experience in infrared spectroscopy.This experiment is a distillation of two different liquids – an alcohol (methanol, ethanol or 1-propanol) and another liquid (acetone, diethyl ether or water). It will be up to you to discoverwhich two liquids are present in the mixture.BACKGROUND:Distillation TechniquesDistillation is a common method used to separate and purify liquids. The process consists ofheating a liquid to its boiling point, condensing the vapours, and collecting the condensate.Figure 1 shows several stages in the evolution of distillation equipment in the organic laboratory.Up until recent years, equipment based on the retort design was common. However, whenperforming microscale experiments, a long distillation path must be avoided. With small samplequantities, there is too much glassware (nooks and crevices, etc.) in a large scale apparatus andtoo many opportunities to lose the entire sample. Microscale equipment based on the retortdesign (A) is expensive and awkward, so we use the Hickman still design (B).Figure 1: The Evolution of Distillation Equipment
General PrinciplesIf a liquid is kept in a sealed container, ner, some molecules escape from the liquid's surface into thespace above it. When equilibrium is established, the number of molecules escaping the liquidequals the number of molecules in the vapour that strike the liquid surface and stick. Themolecules in the vapour also strike the walls of the container and exert a pressure, defined as thevapour pressure of the liquid. If the temperature of the liquid is raised, a greater number ofmolecules escape to the vapour phase until equilibrium is once again established; the vapourpressure of the liquid increases with increasing temperature. This is demonstrated for 4 commonliquids.Figure 2: : Vapour Pressure-Temperature Diagram of 4 liquidsThe boiling point of a liquid is that temperature at which the vapour pressure of the liquid isequal to the pressure of its surroundings. If the flask that contains the liquid is open to theatmosphere, the boiling point will be the temperature at which vapour pressure of the liquid isequal to atmospheric pressure. The vapour pressure of a pure liquid rises steadily as thetemperature is increased, until the boiling point is reached (Figure 2).Simple Distillation1. A Mixture of Ideal LiquidsIf a mixture of two miscible liquids with different boiling points is heated to boiling, the vapourwill not have the same composition as the liquid; it will be richer in the more volatile component.ConsiderFigure 3 (top of the following page), which depicts the behaviour of mixtures of Carbontetrachloride (C) and Toluene (T), two miscible volatile liquids with boiling points BP C and BP T ,respectively. The lower of the two solid curves represents the boiling points of different mixturesof C and T. The upper curve represents the composition of the vapour which is in equilibriumwith the liquid at its boiling point. At 100% C or 100% T the curves meet, because boiling pureC (at BP C ) can have only pure A vapour in equilibrium with it; the same applies to pure T (atBP T ).
Mixtures with a composition to the left of C MIN , in Figure 4A (or C MAX in Figure 4B), can beseparated into pure ethanol (or formic acid in 4B) and the constant boiling mixture, but purewater cannot be obtained from such a mixture by distillation. Conversely, mixtures with acomposition to the right of C MIN , in Figure 4A (or C MAX in 4B), can be separated into pure waterand the constant boiling mixture, but can never furnish pure ethanol/formic acid by distillation.Fractional DistillationIt was noted from Figure 3 that even the first drop of distillate (fraction collected) obtained wasnot pure, but rather a mixture, containing mainly the lower boiling point compound (carbontetrachloride). If these first fractions were to be combined and redistilled, the first vapour to becondensed would be even richer in carbon tetrachloride. Repetition of this process (vaporization,condensation, and revaporization) could eventually lead to isolation of pure carbon tetrachloride.Similar redistillation of the higher-boiling fractions could lead to isolation of pure toluene in thefinal fractions. Clearly this repeated redistillation is a laborious process.Figure 5: : Close-up of the Fractionating Section of a Hickman StillThe fractionating column is a device for increasing the efficiency of this redistillation process. Itconsists of a vertical column packed with some inert material, such as glass beads or glasshelices, or provided with some other device (indentations are used in our apparatus – see Figure5) for increasing the surface upon which the vapour may condense. As the hot vapours risethrough the column, they condense and flow back down the column. The condensate, as it hitsthe lower, hotter portions of the column, is revaporized, and the more volatile componentsproceed up the column once again. This process is repeated numerous times in the fractionatingsection of the Hickman still, and the distillate will consist of the lowest-boiling component in anearly pure form. Figure 6 illustrates the process graphically.
Figure 6: Liquid-Vapour Diagram, illustrating the Principles of FractionalDistillationAn A-B mixture (Figure 6) with composition C 1 boils at temperature T C1 and the vapours enterthe column at that temperature. If they condense in the column, the condensate will have thecomposition C 2 . This vaporizes near the bottom of the column at temperature T C2 , producingvapours with composition C 3 . These may condense further up the column at T C3 ; vaporizationnow gives vapour with composition C 4 , etc. If the column contains enough surface area for manysuccessive vaporizations and condensations (an ideal distillation column), the first distillate thatcomes over will be nearly pure 100% A with a boiling point close to that of pure A. This willcontinue until all of A is removed, after which B will start distilling, and the temperature will riserather abruptly to the boiling point of B. In practice, distilling columns are not 100% efficient,but columns have been designed which can separate liquids that boil as little as 2 o C apart.Thermometer CorrectionGenerally speaking, thermometers may be of two types: total immersion or partial immersion. Ifthe thermometer is not immersed totally or to the immersion line on the thermometer, thetemperature reading will be slightly lower than the true boiling point of your distillate. The stemcorrection may be calculated according to the formula:T c = T o + F x L(T o - T m )where T c = corrected temp.,T o = observed temp.,T m = mean temp. of exposed stem,L = length of exposed mercury column in degrees,F = correction factor (for mercury in pyrex, a value for F of 0.00016 is used).Although you are not expected to perform this correction, keep in mind that your observedtemperature will be lower than the true boiling point, especially for higher temperatures.The Melting Point ApparatusSeveral types of apparatus used to determine melting points are shown below. Types A to C areolder versions and use liquids as the heat transfer agents. In this laboratory you will useapparatus D which comes in two versions: one for use with a capillary tube and the other for use
with a microscope cover slide. You should use the version with capillary tubes. Make sure thatyou use a high temperature (250 or 360 o C) thermometer!PRE-LAB PREPARATION:Read the experimental procedure so that you are prepared for the lab and you understand thesafety and disposal information for the chemicals you are using in this experiment.1. How does vapor pressure determine the boiling point of a mixture (answer in the form of adefinition for boiling point)? What is the point of doing a fractional distillation?2. . (a) Draw the structures of acetone, diethyl ether and water.(b) Using the IR instrument, how would you differentiate these three liquids (include values)?3. . Starting with a mixture of 50% carbon tetrachloride and 50% toluene (see Figures 3 AND 6),and using an IDEAL fractional distillation column, what would be the % composition of toluenein the first distillate (first fraction collected)?EXPERIMENTAL PROCEDURE:THIS LAB WILL BE PERFORMED IN PARTNERS, , but each person must run their ownIR.Safety and Disposal Data for Compounds to be Distilled and Test Reagents.CompoundAcetoneCeric AmmoniumNitrateDiethyl Ether3,5-DinitrobenzoylMol. Wt. (g/mol)58.08548.2374.12230.56Safety and Disposal DataIrritant. Highly Flammable. Dispose in Organic Waste.Irritant. Contact with combustible may cause fire.Wear gloves when handling.Irritant. Extremely flammable. May form explosiveperoxides. Dispose in Organic Waste.Corrosive. Causes burns. Avoid all contact. Wear
HEAT Spa Kur Therapy Development Inc - www.gehwolusa.comTel 707-942-6633 - Fax 707-942-0734www.h-e-a-t.com - email DrB@h-e-a-t.com
your table for up to 10 fractions. Generally the ending temperature of one fraction will be thestarting temperature of the next fraction, except when all of the higher boiling liquid is gone. Atthat time there will be an increase in the temperature before the second liquid starts to collect.Tip: The best way to do this in partners: one person do the collecting of the fractions andparafilming the tubes if necessary, while the other person is in charge of recording thetemperature ranges.Temp. RangeAlcohol Test(Colour)1 stfraction2 nd fraction 3 rd fraction 4 th fraction 5 th fraction 6 th fraction Etc.c) Graph the fractions number (x-axis) against the middle temperature (from the range) for thatfraction.3. Testing the Fractions for Alcohola) Add 1-2 drops of your distillate fraction to 0.5 mL of the yellow Ceric Ammonium Nitratesolution (DO NOT add the yellow Ceric Ammonium Nitrate solution TO your fraction!).b) If an alcohol (ROH) is present, a red colour should develop due to the complex(NH 4 ) 2 Ce[(OR)(NO 3 ) 5 ]. Record the colours and its brightness in the table. i.e. light orange ordark red4. Characterizing your Non-AlcoholBased on your alcohol tests (step 3) and the temperature range of your fractions, you should beable to distinguish the purest fractions of your 2 liquids.a) Take the purest non-alcohol fraction and obtain an IR of your distillate (label pertinent peaks– those above 1600 cm -1 ).b) Based on your IR and boiling point of your distillate (Diethyl Ether 35°C, Acetone 57°C, andwater 100°C), do you have acetone, diethyl ether or water?Remember: the observed BP is lower than the actual BP, especially at higher temperatures.5. Characterizing the Alcohola) Take your purest alcohol fraction and put 0.3 mL of your ‘unknown’ alcohol in a 5 mLconical vial.b) Add 0.1g of 3,5-dinitrobenzoyl chloride 1 and attach a drying tube packed with calciumchloride.c) Heat the vial on a hot plate for 5 minutes, swirling periodically to wash the powder from thewalls of the vial, then let the vial cool to room temperature.d) Add distilled water (not regular tap water) to the 3 mL mark and shake vigorously until youget a precipitate. You want to break up any large chunks of solid (Be careful not to mash yourpowder into the bottom corner of the vial). Cool in an ice bath.1 This is stored in a desiccator. It must be returned to the desiccator after use to prevent loss of activity due to airmoisture.
e) Separate the solid from the solution by vacuum filtration on a Hirsh funnel.The vacuum filtration is done as follows (refer to the diagramon the left). A Hirsch funnel is fitted to a filter flask with aneoprene adapter. A disk of filter paper exactly the correct sizeto cover all the holes in the funnel is placed in the funnel andmoistened with some of the solvent (in this case, water). Thefilter flask is then joined to the vacuum inlet (using thickorange or black tubing) and a vacuum is applied. When thefilter paper is drawn down tightly to the funnel, filtration of thesolution is begun. During filtration, it is important to break thevacuum at either end of the hose connection before turning offthe vacuum. This will prevent loss of powder in the funnel dueto the pressure “suck back”.f) Wash the precipitate twice with 5% sodium bicarbonate (while in the Hirsh funnel, add thesodium bicarbonate and keep filtering) and allow the crystals to dry as much as possible.g) Fill a melting point tube with your solid by thrusting the open end into the solid several times.In order to work the plug of solid material down to the sealed end of the tube, vigorously tap thesealed end on the table or lightly draw a file across the tube held loosely in the hand. Repeat theprocedure until the tube contains a 3 mm column of densely packed powder is in the bottom.h) Place the capillary in the melting point apparatus, turn on the power and allow the hot-stagetemperature to rise fairly rapidly to within 15-20 o C below the expected melting point of thecompound. However, during the determination of the actual melting point range, the temperatureshould not rise more rapidly than 2 or 3 o per minute. A finite time is required to transfer heatfrom the hot-stage both through the walls of the capillary tube and throughout the mass of thesample. If the hot-stage is heated too quickly, its temperature will rise several degrees during thetime lag required for the melting process to occur; this results in an observed range higher thanthe true one.i) Determine the melting point (MP) of the solid derivative (recorded as a RANGE, from whenthe solid starts to melt until it’s done, I know, the name “melting POINT” is deceiving!) and thiswill tell you which alcohol you have. The derivative MPs are as follows: Methanol 108°C,Ethanol 93°C, 1-Propanol 73°C. Based on your derivative test and the boiling point of thedistillate (Methanol 65°C, Ethanol 78°C, 1-Propanol 97°C), which alcohol do you have?Note: Since this is a problem based lab, you will be required to include a conclusion of yourfindings.