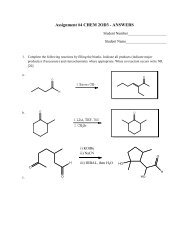
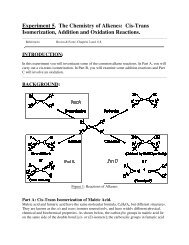
Expt. 2
Expt. 2
Expt. 2
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Figure 6: Liquid-Vapour Diagram, illustrating the Principles of FractionalDistillationAn A-B mixture (Figure 6) with composition C 1 boils at temperature T C1 and the vapours enterthe column at that temperature. If they condense in the column, the condensate will have thecomposition C 2 . This vaporizes near the bottom of the column at temperature T C2 , producingvapours with composition C 3 . These may condense further up the column at T C3 ; vaporizationnow gives vapour with composition C 4 , etc. If the column contains enough surface area for manysuccessive vaporizations and condensations (an ideal distillation column), the first distillate thatcomes over will be nearly pure 100% A with a boiling point close to that of pure A. This willcontinue until all of A is removed, after which B will start distilling, and the temperature will riserather abruptly to the boiling point of B. In practice, distilling columns are not 100% efficient,but columns have been designed which can separate liquids that boil as little as 2 o C apart.Thermometer CorrectionGenerally speaking, thermometers may be of two types: total immersion or partial immersion. Ifthe thermometer is not immersed totally or to the immersion line on the thermometer, thetemperature reading will be slightly lower than the true boiling point of your distillate. The stemcorrection may be calculated according to the formula:T c = T o + F x L(T o - T m )where T c = corrected temp.,T o = observed temp.,T m = mean temp. of exposed stem,L = length of exposed mercury column in degrees,F = correction factor (for mercury in pyrex, a value for F of 0.00016 is used).Although you are not expected to perform this correction, keep in mind that your observedtemperature will be lower than the true boiling point, especially for higher temperatures.The Melting Point ApparatusSeveral types of apparatus used to determine melting points are shown below. Types A to C areolder versions and use liquids as the heat transfer agents. In this laboratory you will useapparatus D which comes in two versions: one for use with a capillary tube and the other for use