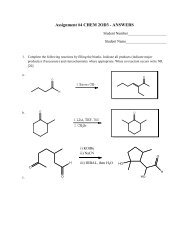
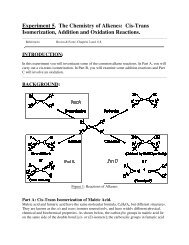
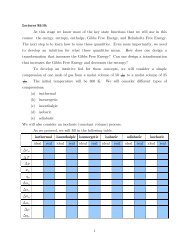
Expt. 2
Expt. 2
Expt. 2
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
with a microscope cover slide. You should use the version with capillary tubes. Make sure thatyou use a high temperature (250 or 360 o C) thermometer!PRE-LAB PREPARATION:Read the experimental procedure so that you are prepared for the lab and you understand thesafety and disposal information for the chemicals you are using in this experiment.1. How does vapor pressure determine the boiling point of a mixture (answer in the form of adefinition for boiling point)? What is the point of doing a fractional distillation?2. . (a) Draw the structures of acetone, diethyl ether and water.(b) Using the IR instrument, how would you differentiate these three liquids (include values)?3. . Starting with a mixture of 50% carbon tetrachloride and 50% toluene (see Figures 3 AND 6),and using an IDEAL fractional distillation column, what would be the % composition of toluenein the first distillate (first fraction collected)?EXPERIMENTAL PROCEDURE:THIS LAB WILL BE PERFORMED IN PARTNERS, , but each person must run their ownIR.Safety and Disposal Data for Compounds to be Distilled and Test Reagents.CompoundAcetoneCeric AmmoniumNitrateDiethyl Ether3,5-DinitrobenzoylMol. Wt. (g/mol)58.08548.2374.12230.56Safety and Disposal DataIrritant. Highly Flammable. Dispose in Organic Waste.Irritant. Contact with combustible may cause fire.Wear gloves when handling.Irritant. Extremely flammable. May form explosiveperoxides. Dispose in Organic Waste.Corrosive. Causes burns. Avoid all contact. Wear