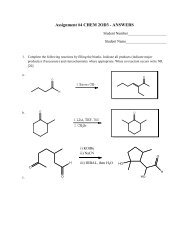
Expt. 2
Expt. 2
Expt. 2
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
your table for up to 10 fractions. Generally the ending temperature of one fraction will be thestarting temperature of the next fraction, except when all of the higher boiling liquid is gone. Atthat time there will be an increase in the temperature before the second liquid starts to collect.Tip: The best way to do this in partners: one person do the collecting of the fractions andparafilming the tubes if necessary, while the other person is in charge of recording thetemperature ranges.Temp. RangeAlcohol Test(Colour)1 stfraction2 nd fraction 3 rd fraction 4 th fraction 5 th fraction 6 th fraction Etc.c) Graph the fractions number (x-axis) against the middle temperature (from the range) for thatfraction.3. Testing the Fractions for Alcohola) Add 1-2 drops of your distillate fraction to 0.5 mL of the yellow Ceric Ammonium Nitratesolution (DO NOT add the yellow Ceric Ammonium Nitrate solution TO your fraction!).b) If an alcohol (ROH) is present, a red colour should develop due to the complex(NH 4 ) 2 Ce[(OR)(NO 3 ) 5 ]. Record the colours and its brightness in the table. i.e. light orange ordark red4. Characterizing your Non-AlcoholBased on your alcohol tests (step 3) and the temperature range of your fractions, you should beable to distinguish the purest fractions of your 2 liquids.a) Take the purest non-alcohol fraction and obtain an IR of your distillate (label pertinent peaks– those above 1600 cm -1 ).b) Based on your IR and boiling point of your distillate (Diethyl Ether 35°C, Acetone 57°C, andwater 100°C), do you have acetone, diethyl ether or water?Remember: the observed BP is lower than the actual BP, especially at higher temperatures.5. Characterizing the Alcohola) Take your purest alcohol fraction and put 0.3 mL of your ‘unknown’ alcohol in a 5 mLconical vial.b) Add 0.1g of 3,5-dinitrobenzoyl chloride 1 and attach a drying tube packed with calciumchloride.c) Heat the vial on a hot plate for 5 minutes, swirling periodically to wash the powder from thewalls of the vial, then let the vial cool to room temperature.d) Add distilled water (not regular tap water) to the 3 mL mark and shake vigorously until youget a precipitate. You want to break up any large chunks of solid (Be careful not to mash yourpowder into the bottom corner of the vial). Cool in an ice bath.1 This is stored in a desiccator. It must be returned to the desiccator after use to prevent loss of activity due to airmoisture.