Expt. 2
Expt. 2
Expt. 2
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
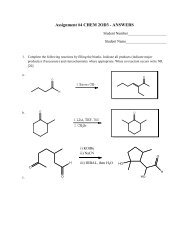
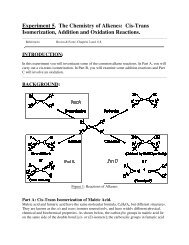
e) Separate the solid from the solution by vacuum filtration on a Hirsh funnel.The vacuum filtration is done as follows (refer to the diagramon the left). A Hirsch funnel is fitted to a filter flask with aneoprene adapter. A disk of filter paper exactly the correct sizeto cover all the holes in the funnel is placed in the funnel andmoistened with some of the solvent (in this case, water). Thefilter flask is then joined to the vacuum inlet (using thickorange or black tubing) and a vacuum is applied. When thefilter paper is drawn down tightly to the funnel, filtration of thesolution is begun. During filtration, it is important to break thevacuum at either end of the hose connection before turning offthe vacuum. This will prevent loss of powder in the funnel dueto the pressure “suck back”.f) Wash the precipitate twice with 5% sodium bicarbonate (while in the Hirsh funnel, add thesodium bicarbonate and keep filtering) and allow the crystals to dry as much as possible.g) Fill a melting point tube with your solid by thrusting the open end into the solid several times.In order to work the plug of solid material down to the sealed end of the tube, vigorously tap thesealed end on the table or lightly draw a file across the tube held loosely in the hand. Repeat theprocedure until the tube contains a 3 mm column of densely packed powder is in the bottom.h) Place the capillary in the melting point apparatus, turn on the power and allow the hot-stagetemperature to rise fairly rapidly to within 15-20 o C below the expected melting point of thecompound. However, during the determination of the actual melting point range, the temperatureshould not rise more rapidly than 2 or 3 o per minute. A finite time is required to transfer heatfrom the hot-stage both through the walls of the capillary tube and throughout the mass of thesample. If the hot-stage is heated too quickly, its temperature will rise several degrees during thetime lag required for the melting process to occur; this results in an observed range higher thanthe true one.i) Determine the melting point (MP) of the solid derivative (recorded as a RANGE, from whenthe solid starts to melt until it’s done, I know, the name “melting POINT” is deceiving!) and thiswill tell you which alcohol you have. The derivative MPs are as follows: Methanol 108°C,Ethanol 93°C, 1-Propanol 73°C. Based on your derivative test and the boiling point of thedistillate (Methanol 65°C, Ethanol 78°C, 1-Propanol 97°C), which alcohol do you have?Note: Since this is a problem based lab, you will be required to include a conclusion of yourfindings.