INFUSE® Bone Graft - BioHorizons
INFUSE® Bone Graft - BioHorizons
INFUSE® Bone Graft - BioHorizons
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
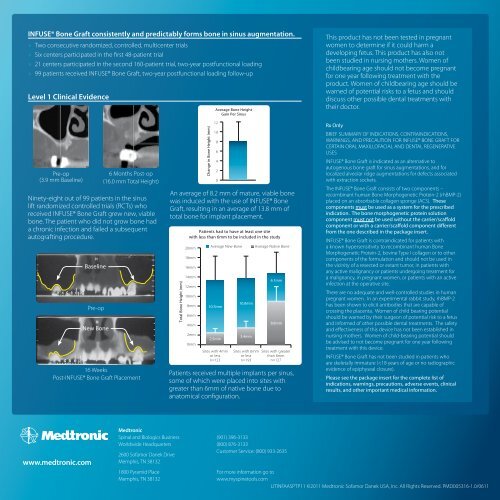
INFUSE® <strong>Bone</strong> <strong>Graft</strong> consistently and predictably forms bone in sinus augmentation.»»Two consecutive randomized, controlled, multicenter trials»»Six centers participated in the first 48-patient trial»»21 centers participated in the second 160-patient trial, two-year postfunctional loading»»99 patients received INFUSE® <strong>Bone</strong> <strong>Graft</strong>, two-year postfunctional loading follow‐upLevel 1 Clinical EvidenceAverage <strong>Bone</strong> HeightGain Per SinusThis product has not been tested in pregnantwomen to determine if it could harm adeveloping fetus. This product has also notbeen studied in nursing mothers. Women ofchildbearing age should not become pregnantfor one year following treatment with theproduct. Women of childbearing age should bewarned of potential risks to a fetus and shoulddiscuss other possible dental treatments withtheir doctor.Pre-op(3.9 mm Baseline)Ninety-eight out of 99 patients in the sinuslift randomized controlled trials (RCTs) whoreceived INFUSE® <strong>Bone</strong> <strong>Graft</strong> grew new, viablebone. The patient who did not grow bone hada chronic infection and failed a subsequentautografting procedure.BaselinePre-opNew <strong>Bone</strong>6 Months Post-op(16.0 mm Total Height)16 WeeksPost-INFUSE® <strong>Bone</strong> <strong>Graft</strong> PlacementTotal <strong>Bone</strong> Height (mm)Change in <strong>Bone</strong> Height (mm)121086420An average of 8.2 mm of mature, viable bonewas induced with the use of INFUSE® <strong>Bone</strong><strong>Graft</strong>, resulting in an average of 13.8 mm oftotal bone for implant placement.Patients had to have at least one sitewith less than 6mm to be included in the study20mm18mm16mm14mmAverage New <strong>Bone</strong> Average Native <strong>Bone</strong>6.1mm12mm10mm8mm6mm10.7mm10.0mm4mm8.8mm2mm0mm2.5mm3.4mmSites with 4mmor lessn=123Sites with 6mmor lessn=193Sites with greaterthan 6mmn=127Patients received multiple implants per sinus,some of which were placed into sites withgreater than 6mm of native bone due toanatomical configuration.Rx OnlyBRIEF SUMMARY OF INDICATIONS, CONTRAINDICATIONS,WARNINGS, AND PRECAUTION FOR INFUSE® BONE GRAFT FORCERTAIN ORAL MAXILLOFACIAL AND DENTAL REGENERATIVEUSESINFUSE® <strong>Bone</strong> <strong>Graft</strong> is indicated as an alternative toautogenous bone graft for sinus augmentations, and forlocalized alveolar ridge augmentations for defects associatedwith extraction sockets.The INFUSE® <strong>Bone</strong> <strong>Graft</strong> consists of two components –recombinant human <strong>Bone</strong> Morphogenetic Protein-2 (rhBMP-2)placed on an absorbable collagen sponge (ACS). Thesecomponents must be used as a system for the prescribedindication. The bone morphogenetic protein solutioncomponent must not be used without the carrier/scaffoldcomponent or with a carrier/scaffold component differentfrom the one described in the package insert.INFUSE® <strong>Bone</strong> <strong>Graft</strong> is contraindicated for patients witha known hypersensitivity to recombinant human <strong>Bone</strong>Morphogenetic Protein-2, bovine Type I collagen or to othercomponents of the formulation and should not be used inthe vicinity of a resected or extant tumor, in patients withany active malignancy or patients undergoing treatment fora malignancy, in pregnant women, or patients with an activeinfection at the operative site.There are no adequate and well-controlled studies in humanpregnant women. In an experimental rabbit study, rhBMP-2has been shown to elicit antibodies that are capable ofcrossing the placenta. Women of child bearing potentialshould be warned by their surgeon of potential risk to a fetusand informed of other possible dental treatments. The safetyand effectiveness of this device has not been established innursing mothers. Women of child-bearing potential shouldbe advised to not become pregnant for one year followingtreatment with this device.INFUSE® <strong>Bone</strong> <strong>Graft</strong> has not been studied in patients whoare skeletally immature (