Ultramid A34 01 - BASF Plastics Portal
Ultramid A34 01 - BASF Plastics Portal
Ultramid A34 01 - BASF Plastics Portal
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
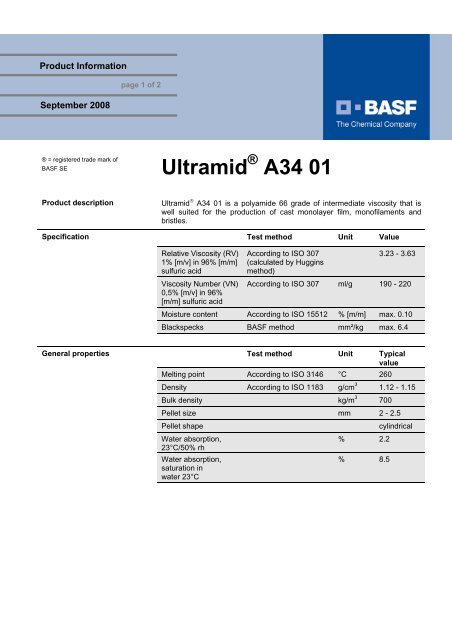
0Product InformationSeptember 2008page 1 of 2® = registered trade mark of<strong>BASF</strong> SE<strong>Ultramid</strong> ® <strong>A34</strong> <strong>01</strong>Product description<strong>Ultramid</strong> ® <strong>A34</strong> <strong>01</strong> is a polyamide 66 grade of intermediate viscosity that iswell suited for the production of cast monolayer film, monofilaments andbristles.Specification Test method Unit ValueRelative Viscosity (RV)1% [m/v] in 96% [m/m]sulfuric acidViscosity Number (VN)0,5% [m/v] in 96%[m/m] sulfuric acidAccording to ISO 307(calculated by Hugginsmethod)3.23 - 3.63According to ISO 307 ml/g 190 - 220Moisture content According to ISO 15512 % [m/m] max. 0.10Blackspecks <strong>BASF</strong> method mm²/kg max. 6.4General properties Test method Unit TypicalvalueMelting point According to ISO 3146 °C 260Density According to ISO 1183 g/cm 3 1.12 - 1.15Bulk density kg/m 3 700Pellet size mm 2 - 2.5Pellet shapeWater absorption,23°C/50% rhWater absorption,saturation inwater 23°C% 2.2% 8.5cylindrical
September 2008 page 2 of 2 <strong>Ultramid</strong> ® <strong>A34</strong> <strong>01</strong>Supply form and storageFood legislationDisclaimerMedical disclaimer<strong>Ultramid</strong> ® <strong>A34</strong> <strong>01</strong> is supplied pre-dried and ready for processing in a varietyof moisture proof containers, such as boxes, bigbags (Asia) and bulk containers.The material must be protected against moisture during storage. Astorage time of 6 months should not be exceeded. Opened bags should beused up immediately in order to prevent moisture pickup.Certificates regarding the status of <strong>Ultramid</strong> ® <strong>A34</strong> <strong>01</strong> with respect to the FDARegulation 21 CFR 177.1500 "Nylon Resins", the European Regulation2002/72/EC, the German BfR recommendation "X.Polyamide", 1.6.1998 (Bedarfsgegenständeverordnungin der Neufassung vom 23.12.97 einschließlichder neunten Verordnung zur Änderung der Bedarfsgegenständeverordnung vom 07.04.2003) or legislations for other countries will beprovided on request.While the descriptions, designs, data and information contained herein arepresented in good faith and believed to be accurate, it is provided for yourguidance only. Because many factors may affect processing or application/use,we recommend that you make tests to determine the suitability of aproduct for your particular purpose prior to use. NO WARRANTIES OF ANYKIND, EITHER EXPRESS OR IMPLIED, INCLUDING WARRANTIES OFMERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE, AREMADE REGARDING PRODUCTS DESCRIBED OR DESIGNS, DATA ORINFORMATION SET FORTH, OR THAT THE PRODUCTS, DESIGNS, DATAOR INFORMATION MAY BE USED WITHOUT INFRINGING THEINTELLECTUAL PROPERTY RIGHTS OF OTHERS. IN NO CASE SHALLTHE DESCRIPTIONS, INFORMATION, DATA OR DESIGNS PROVIDED BECONSIDERED A PART OF OUR TERMS AND CONDITIONS OF SALE.Further, you expressly understand and agree that the descriptions, designs,data and information furnished by <strong>BASF</strong> hereunder are provided gratis and<strong>BASF</strong> assumes no obligation or liability for the description, designs, data andinformation given or results obtained, all such being given and accepted atyour risk.<strong>BASF</strong> has not developed or tested its plastics especially for the use in medicaldevices (defined in risk classes I to III according to the European and USMedical Device legislation) and pharmaceutical applications. Therefore <strong>BASF</strong>makes no warranties, express or implied, concerning the suitability of any<strong>BASF</strong> plastics for use in any medical device and pharmaceutical applications.<strong>BASF</strong> does not supply its plastics for the manufacture of implants of any riskclass.Please inform us in advance, if you intend to use <strong>BASF</strong> plastics in medicaldevices or pharmaceutical applications.Further information Europe: www.basf.deextrusion.ultramid@basf.comTel.: +49 621 60 42888NAFTA:Asia:www.basf.comwww.plasticsportal.comwww.basf.compolymer-hk@basf.comTel.: +1 800 527 8324Tel.: +852 2731 1247