You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
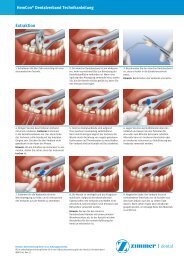
<strong>English</strong> Rev 0-07/11INSTRUCTIONS FOR USEZIMMER ® ANGLED TAPERED ABUTMENTS FOR TAPERED SCREW-VENT ® AND SCREW-VENT ® IMPLANT SYSTEMSBefore using a <strong>Zimmer</strong> <strong>Dental</strong> product, the operating surgeon/practitioner in charge should carefully study the indications,contraindications, recommendations, warnings and instructions, as well as all other product-specific information (technicalproduct description, description of the surgical and restorative technique, catalogue sheet, etc.) and fully comply with them.Detailed instructions over and above those contained in this instruction for use concerning the possible combinations, productspecificrisks, preparatory steps, indications and contraindications, etc. can be found in the description of the surgicaltechnique, in the technical description of the product or on the appropriate catalogue sheet. <strong>Zimmer</strong> also recommendsattending the appropriate user-training courses. The aforementioned documents and details of the training courses may beobtained from the appropriate representatives in the various countries. The manufacturer, the importer and the suppliers of<strong>Zimmer</strong> products are not liable for complications, other negative effects or damages that might occur for reasons such asincorrect indications or surgical technique, unsuitable choice of material or handling thereof, unsuitable use or handling of theinstruments, asepsis and so on. The operating surgeon is responsible for any such complications or other consequences. It isalso the operating surgeon's responsibility to properly instruct and inform the patient on the functions, handling and necessarycare of the product and on all known product risks.DESCRIPTIONThe Angled Tapered abutment has a hex which engages the internal hex of the Tapered Screw-Vent and Screw-Vent implant.The abutments are available in multiple cuff heights and in both 15° and 30° configurations to provide angulation correction foroff-angle implant placement. The abutment is secured to the implant with an abutment retaining screw which is preassembledin the abutment. The abutment screw is not removable from the abutment. The abutment has a tapered cone with an internalscrew access for the attachment of various restorative components using a separate coping screw. The abutment cone has a15° taper and is 1.2mm tall. Abutments are packaged with a metal delivery tool to assist with the placement and orientation ofthe abutment into the implant. The abutment and abutment retaining screw are fabricated from titanium alloy.INDICATIONSThe Angled Tapered Abutment is used for a terminal or intermediate abutment for screw-retained multiple-unit restorations.The 30° Angled Tapered Abutment must be used within 45° of parallelism for a splinted restoration. The 15° Angled TaperedAbutment must be used within 30° of parallelism for a splinted restoration.CONTRAINDICATIONSNot for use as a single tooth restoration, with a cemented prosthesis, or with limited interocclusal space less than 7.5mm. Notfor use when implants are divergent greater than 45° with the 30° Angled Tapered Abutment or when implants are divergentgreater than 30° with the 15° Angled Tapered Abutment. The Angled Tapered Abutment may not be prepped. The AngledTapered Abutment is not for use with the 3.3mm Screw-Vent Implant.<strong>Zimmer</strong> <strong>Dental</strong> implants should not be placed if there is an insufficient volume of alveolar bone to minimally support the implant(minimum 1mm circumferential and 2mm apical). Implants placed in the maxilla should not perforate the sinus floor membrane.Poor bone quality, poor patient oral hygiene, heavy tobacco use, uncontrolled systematic diseases (diabetes, etc.), reducedimmunity, alcoholism, drug addiction, and psychological instability may contribute to lack of integration and/or subsequentimplant failure. Severe bruxism, clenching, and overloading, may cause bone loss, screw loosening, component fracture,and/or implant failure. Exposure to radiation and chemotherapy may impact health of the implant. <strong>Dental</strong> implant patientsshould be instructed to consult with their physician prior to undergoing such treatment options.WARNINGSSurgical and restorative techniques required to place dental implants are highly specialized and complex procedures.Practitioners should attend courses of study to familiarize themselves with implantology techniques. Improper technique cancause bone loss and implant failure. <strong>Zimmer</strong> <strong>Dental</strong> implant systems are intended to be used only with <strong>Zimmer</strong> <strong>Dental</strong> speciallydesigned bone drills and prosthetics. Implants placed at severe angles relative to existing dentition or multiple implants placedat convergent/divergent manner can result in complex restorations that may overload implants, potentially leading to implantfailure. A thorough diagnostic work-up and use of surgical templates are recommended to help ensure proper angulation.Other relative contraindications include steroid and anticoagulant treatment which may affect the surgical site, surroundingtissue, or patient’s healing function.Exposure to long-term use of bisphosphonate drugs especially with chemotherapy mayimpact implant survival. Careful patient selection including consultation with the attending physician is strongly recommendedprior to implant treatment.Excessive mobility, bone loss, or infection may indicate the implant is failing. Any implant whichappears to be failing should be treated or removed as soon as possible. If removal is necessary, curette any soft tissue fromthe implant site and allow site to heal as though it were an atraumatic extraction.Due to the metal conductivity, electrosurgeryaround the implants and intraoral abutment preparations without irrigation could result in tissue damage and implant failure.Patients should consult with their physician and imaging technician prior to undergoing an MRI procedure. The Angled TaperedAbutment has not been evaluated for safety and compatibility in the MR environment. The Angled Tapered Abutment has notbeen tested for heating or migration in the MR environment.PRECAUTIONSProper case planning is essential to the long-term success of both the prosthesis and the implant. Overload is one of the keycontributors to implant failure. Ensure the implant size and abutment angulation are appropriate for the occlusal load. Highlyangulated abutments (>30°) should be avoided. Off Axis Loading – if implants are placed up to 45° off axis then it may benecessary to splint(join the restoration) the divergent implants.IFU 9419 Final Document
<strong>English</strong> Rev 0-07/11BREAKAGEImplant and abutment fractures can occur when applied loads exceed the normal functional design tolerances of the implantcomponents. Potential overloading conditions may result from deficiencies in implant numbers, lengths and/or diameters toadequately support a restoration, excessive cantilever length, incomplete abutment seating, abutment angles greater than 30degrees, occlusal interferences causing excessive lateral forces, patient parafunction (e.g., bruxing clenching), impropercasting procedures, inadequate prosthesis fit, and physical trauma.CHANGES IN PERFORMANCEIt is the responsibility of the clinician to instruct the patient on all appropriate contraindications, side effects, and precautions aswell as the need to seek the services of a trained dental professional if there are any changes in the performance of the implant(e.g., looseness of the prosthesis, infection or exudate around the implant, pain, or any other unusual symptoms that thepatient has not been told to expect).HYGIENE & MAINTENANCELong-term implant health is directly related to the maintenance of oral hygiene. Potential implant candidates should establishan adequate oral hygiene regimen prior to implant therapy. Following implant placement, the clinician should instruct thepatient on proper tools and techniques to ensure long-term maintenance of the implant(s). The patient should also beinstructed to maintain routinely scheduled prophylaxis and evaluation appointments.TREATMENT PLANNINGAppropriate imaging techniques should be used to determine if adequate bone is available, and to determine the location ofimportant anatomical landmarks, such as the mandibular canal, maxillary sinuses and adjacent teeth. Thorough clinicalevaluation is imperative prior to all implant surgeries.GENERAL CONSIDERATIONSControl of biomechanical stresses is the key factor to long-term success of the prosthesis. Even after implant integration,imbalances in occlusal forces can lead to implant failure. Implant patients should be monitored for signs of screw loosening,periimplant bone loss and tooth wear as signs of occlusal overloading.ADVERSE EFFECTSThe following complications may occur relative to implant placement: pain, discomfort, dehiscence, delayed healing,paresthesia, hyperesthesia, edema, hemorrhage, hematoma, infection, inflammation, local and generalized allergic reaction,lack of integration, loss of bone, and loss of implant. Other adverse effects may also occur as a result of iatrogenic factors andhost responses.STERILITYAll implants have been gamma radiation sterilized and are for single use only. Do not resterilize implants. <strong>Zimmer</strong> <strong>Dental</strong>prosthetic and ancillary components are sold sterile or non-sterile. Refer to the specific packaging for verification of sterility.Sterilize non-sterile product prior to use in patients.SINGLE USEReuse of a single use device that has come in contact with blood, bone, tissue or other body fluids may lead to patient or userinjury. Possible risks associated with reuse of a single use device include, but are not limited to, mechanical failure andtransmission of infectious agents.SHELF LIFEThe product expiration date is indicated by the hourglass symbol on the product label, followed by the year and month ofexpiration. Caution: Do not use sterile devices if the packaging providing the sterile barrier, including the outer cap, vial, or trayhas been damaged or compromised in any manner (i.e. cracked or crushed).PRODUCT PACKAGINGAll implants have been cleaned, packaged in double vials within an environmentally controlled room, and sterilized forconvenience and immediate use. The implants are suspended on a carrier for transfer to the prepared surgical site without riskof contact contamination. Both the implant and the inner vial packaging are sterile. The label on the outer vial packaging foreach implant contains a lot number that should be recorded in the patient’s file to ensure complete traceability of the product.Prosthetic components provided in sealed plastic vials or Tyvek ®* sealed blister packages are also pre-cleaned and sterilizedfor your convenience.CLEANING/STERILIZATION INFORMATIONDisinfection and sterilization procedures should conform to OSHA or local guidelines for blood borne pathogens. Clinicallycontaminated implants should not be cleaned and resterilized under any circumstances.CLEANINGUse the following guidelines for cleaning products:Drills, Instruments and Components - Disassemble multi-piece components, if applicable. Rinse with cool-to-lukewarmwater for two-and-one-half minutes. For Drills, use the <strong>Zimmer</strong> cleaning wire to remove any debris from the irrigation channel.Using a 25 gauge needle, flush the drill lumen with water to remove any remaining debris. For all parts place in an ultrasoniccleaner with an enzymatic detergent diluted with tap water per the manufacture’s guidelines. Sonicate for 10 minutes. Rinsewith tap water for three minutes.Kits, Trays, and Blocks - Remove all parts and insert from the tray. Clean parts per the above instructions. Thoroughly rinsethe kits under running tap water to remove all visible soil. Use a soft bristle brush to clean the kits until all visible soil isremoved. A syringe or pipe cleaner may be used to aid in the rinsing. Assure that all hard to reach areas are accessed. Afterthe rinsing, prepare the enzymatic detergent following the manufacturer’s specifications. Fully immerse the kit in the preparedIFU 9419 Final Document
<strong>English</strong> Rev 0-07/11detergent and allow the kit to soak in the detergent for a minimum of one minute. Following the soak use a damp cloth and/or asoft bristle brush to wipe and remove any excess debris/soil from each component. A syringe or a pipe cleaner may be used toaid in the cleaning. Rinse the kits with lukewarm tap water to eliminate all residual enzymes and detergent, thoroughly for aminimum of three minutes. Dry the components. Reassemble the contents of the kit and follow the guidelines for sterilization.Note: This procedure should be performed after an instrument used during a surgery comes into contact with the surgical trayor prosthetic tray.STERILIZATIONIndividual parts should be placed in appropriate autoclave or dry heat pouch prior to sterilization. When sterilizing parts within akit, parts should be placed in appropriate locations and kit should be wrapped in sterilization wrap. The following sterilizationparameters (method, time and temperature) are required to achieve a 10 -6 sterility assurance level (SAL). Local or nationalspecifications should be followed where steam sterilization requirements are stricter or more conservative than those listed inthe table. Exceeding these sterilization parameters may result in damage to plastic components. Verify the calibration of yourunit to ensure recommended temperatures are not being exceeded. To ensure autoclave is performing effectively, periodic useof biologic indicators should be considered. Chemclave sterilization is NOT recommended for any <strong>Zimmer</strong> <strong>Dental</strong> products.PartsindividuallypouchedCycle Type Temperature Exposure TimeGravity(steam)121°C250°FDry Time(only for kits)40 minutes n/aGravity(steam)121°C250°F80 minutes 30 minutesParts individuallypouched or in kitPre-vacuum(steam)Pre-vacuum(steam)Dry Heat132°C270°F134°C273°F160°C320°F3 minutes 30 minutes18 minutes 30 minutes120 minutes n/aTECHNIQUE INFORMATIONProcedure for Angled Tapered AbutmentsNote: During implant placement, it is recommended to orient the flat of the internal hex of the implant to be opposite the anglecorrection. The pre-attached multi-purpose fixture mount can be used to index the internal hex of the implant. The flat side onthe wall of the fixture mount will line up with the flat side of the internal hex.Note: Prior to delivering the abutment to the mouth with the abutment delivery tool, the delivery tool should be hand tightenedto the abutment to confirm adequate attachment of the tool to the abutment.Note: Use appropriate abutments and ancillary components that correspond to the implant system being restored.1. Remove the Angled Tapered Abutment from the abutment packaging over a sterile field. Hand tighten the abutmentdelivery tool to confirm attachment to the cone of the abutment.2. Thread dental floss thru the floss hole in the delivery tool and tie. Utilizing the abutment delivery tool, deliver theabutment to the mouth, aligning the angled abutment in the appropriate orientation for desired angulation correction.3. Use a 1.25mm (0.50”) Hex Driver to hand tighten the abutment retaining screw. A contra angle hand piece with a1.25mmD latch-lock driver may also be used for initial delivery. The long latch-lock driver (part# HXL1.25D) must beused if the abutment delivery tool is attached to the abutment. The standard latch-lock driver (part# HX1.25D) canbe used if the abutment delivery tool is removed from the abutment.4. Verify with periapical radiograph that the abutment is seated completely into the implant and has engaged theinternal hexagon.5. Tighten the abutment retaining screw to 30 Ncm with a calibrated torque wrench. If using the <strong>Zimmer</strong> RestorativeTorque Wrench (part# TWR), the long Torque Wrench Insert Hex Driver, (part# TW1.25L) must be used if theabutment delivery tool is attached to the abutment. The short Torque Wrench Insert Hex Driver (part# TW1.25) canbe used if the abutment delivery tool is removed from the abutment.IFU 9419 Final Document
<strong>English</strong> Rev 0-07/116. If the abutments will not be immediately restored with a provisional or final restoration, it t is recommended to placethe Tapered Abutment Titanium Healing Cap (part# TATHC) to prevent irritation of the soft tissue and to prevent theingress of material in the screw access of the abutment cone.Labeling SymbolsSymbols may be used on some international package labeling for easy identification.SymbolsUse of SymbolsSymbol for “European Conformity”0197Symbol for Angled Abutment CuffHeightSymbol for “Do Not Reuse”Symbol for “Attention seeinstructions for use”Symbol for “Sterile” by gammairradiationSymbol for “Use By”Symbol for HexagonSymbol for “Use by Prescriptiononly”Authorized Representative in theEuropean CommunityCONTACT AND ORDERING INFORMATIONUSA:<strong>Zimmer</strong> <strong>Dental</strong> Inc.1900 Aston AvenueCarlsbad, CA 92008France<strong>Zimmer</strong> <strong>Dental</strong>SAS2,Place GustaveEiffel94528 RungisCedexSpain:<strong>Zimmer</strong> <strong>Dental</strong>Ibérica S.L.U.C/Verge del Pilar49,local 2-308440 CardedeuTel: 800-854-6691, 760-929-4300 FranceTel: +33-(0)1-45-(Barcelona) SpainTel: + 34-93-846-Fax: 760-431-781112-35350543Fax: +33-(0)1-45-Fax: + 34-93-845-60-04884325Canada:<strong>Zimmer</strong> <strong>Dental</strong> Corp. Germany:2323 Argentia RoadMississauga, Ontario, L5N 5N3Tel: 1-800-265-09681-905-567-2073<strong>Zimmer</strong> <strong>Dental</strong>GmbH Italy: <strong>Zimmer</strong> <strong>Dental</strong>Wentzinger Strasse23 Viale Matteotti 9131029 Vittorio VenetoD-79106 Freiburg(TV)Tel: +49 761 / 1564 70 ItalyFax: +49 761 / 15 Tel: + 39-043-855 55IFU 9419 Final Document
Fax: 1-905-567-207664 74 90 73Fax: + 39-043-855 3181<strong>English</strong> Rev 0-07/11Australia: <strong>Zimmer</strong> <strong>Dental</strong> Israel:<strong>Zimmer</strong> <strong>Dental</strong>Ltd.Units 1-2, 1 SkylinePlace143 Bialik StreetFrenchs Forest NSW2086 Ramat Gan 52523Tel: +61(0)2-9950-5444 orTel: +972-(0)3612-4242(NZ) 0-800-305-566Fax: +972-(0) 3612-4243MAH-TSV& SP:Fax: 02-9975-3594Implatex Co. LtdYDM Nippori Bldg 33-19MAH-Spline &AdVent :HakuhoCorporationKojimachi 1,2 chome Nishi Nippori Chome Sanbanchi Bldg. 202Arakawa-ku, Tokyo116-0013 Kojimachi 1-3-23,Japan Chiyoda-ku, Tokyo 102-0083Tel: +813-5850-8555Fax: +813-5850-8505JapanTel: +81-3-3265-6251Fax: +81-3-3263-7316** Tyvek ® Pouch is a registered trademark of E.I. du Pont de Nemours and Company. All other names and logos, andtrademarks referred to within this package insert are the property of <strong>Zimmer</strong> Inc. or its affiliates.Rev. 0-07/11 (and date of printing)Printed in U.S.A. (if applicable)©2011 <strong>Zimmer</strong>, Inc. All rights reserved.(Reflects date of current revision and first release of insert/IFU) 87-620X-XXX-XX or D 011 500 xxx0197 The CE mark is valid only if it is also printed on the product label.IFU 9419 Final Document