Meeting the Requirements of the FDA Food Code Variance in ...
Meeting the Requirements of the FDA Food Code Variance in ...
Meeting the Requirements of the FDA Food Code Variance in ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Page 41 <strong>of</strong> 146<strong>Meet<strong>in</strong>g</strong> <strong>the</strong> <strong>Requirements</strong> <strong>of</strong> <strong>the</strong> <strong>FDA</strong> <strong>Food</strong> <strong>Code</strong> <strong>Variance</strong> <strong>in</strong> Relation to Specialized Meat and Poultry Process<strong>in</strong>g Methods
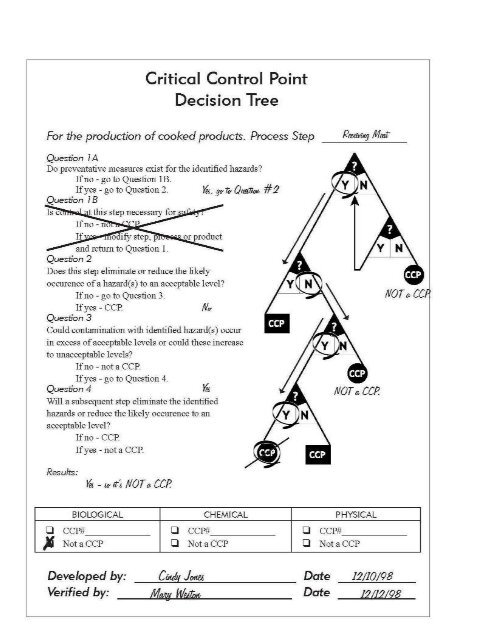
The second step <strong>the</strong>y looked at was cook<strong>in</strong>g.Question 1aThe Example Facility answered “Yes” here because <strong>the</strong>y had identified <strong>the</strong> preventive measure <strong>of</strong> cook<strong>in</strong>g (i.e. timeand temperature) for this step.Question 1bAs <strong>in</strong> <strong>the</strong> receiv<strong>in</strong>g example, move onto question 2.Question 2The Example Facility said that “Yes” cook<strong>in</strong>g would elim<strong>in</strong>ate <strong>the</strong> hazard at this step. They stopped here atquestion 2 because <strong>the</strong>y reached a positive result...<strong>the</strong>ir CCP. Thus, <strong>the</strong>re wasn’t any need to go on to questions3 and 4.[After f<strong>in</strong>d<strong>in</strong>g all <strong>the</strong> CCP’s <strong>in</strong> your process, <strong>the</strong> HACCP team needs to organize <strong>the</strong>m. At <strong>the</strong> bottom <strong>of</strong> <strong>the</strong> CCPDecision Tree Form <strong>the</strong> Example Facility named <strong>the</strong> cook<strong>in</strong>g CCP “CCP#01B”. The “01” tells <strong>the</strong>m what number<strong>the</strong> CCP is, and <strong>the</strong> “B” tells <strong>the</strong>m it is a biological food safety hazard.]Page 42 <strong>of</strong> 146<strong>Meet<strong>in</strong>g</strong> <strong>the</strong> <strong>Requirements</strong> <strong>of</strong> <strong>the</strong> <strong>FDA</strong> <strong>Food</strong> <strong>Code</strong> <strong>Variance</strong> <strong>in</strong> Relation to Specialized Meat and Poultry Process<strong>in</strong>g Methods
Page 43 <strong>of</strong> 146<strong>Meet<strong>in</strong>g</strong> <strong>the</strong> <strong>Requirements</strong> <strong>of</strong> <strong>the</strong> <strong>FDA</strong> <strong>Food</strong> <strong>Code</strong> <strong>Variance</strong> <strong>in</strong> Relation to Specialized Meat and Poultry Process<strong>in</strong>g Methods
Page 44 <strong>of</strong> 146<strong>Meet<strong>in</strong>g</strong> <strong>the</strong> <strong>Requirements</strong> <strong>of</strong> <strong>the</strong> <strong>FDA</strong> <strong>Food</strong> <strong>Code</strong> <strong>Variance</strong> <strong>in</strong> Relation to Specialized Meat and Poultry Process<strong>in</strong>g Methods
Pr<strong>in</strong>ciple 3: Establish Critical Limits for Each Critical Control Po<strong>in</strong>tA critical limit is def<strong>in</strong>ed as “The maximum or m<strong>in</strong>imum value to which a physical, biological, or chemical hazardmust be controlled at a critical control po<strong>in</strong>t to prevent, elim<strong>in</strong>ate, or reduce to an acceptable level <strong>the</strong>occurrence <strong>of</strong> <strong>the</strong> identified food safety hazard.” You can th<strong>in</strong>k <strong>of</strong> a critical limit as a boundary <strong>of</strong> safety for aCCP. The critical limit is <strong>the</strong> numerical value that must be reached to assure that hazards have been controlled.An example would be that “all sausage products must be cooked to 155 o F for 15 seconds.”Each CCP will have at least one (possibly more) preventive measures that need to be controlled to assure thisprevention, elim<strong>in</strong>ation or reduction <strong>of</strong> food safety hazards. To be effective, each critical limit should be:1. Based on proven factual <strong>in</strong>formation. A few ways that <strong>in</strong>formation and recommendations forappropriate limits can be obta<strong>in</strong>ed are: from regulatory requirements, scientific literature, andconsultation with experts. If regulatory requirements exist <strong>the</strong>y must be met or exceeded.2. Objectives are measurable or observable, such as time and temperature.3. Appropriate and reasonable for <strong>the</strong> food product and operation. You should consider <strong>the</strong> type <strong>of</strong>equipment, <strong>the</strong> volume <strong>of</strong> product be<strong>in</strong>g produced, how <strong>the</strong> critical limit will be monitored andfrequency <strong>of</strong> monitor<strong>in</strong>g.4. Specifics. When draft<strong>in</strong>g your critical limits be specific <strong>in</strong> your language. Use action words, and bespecific when nam<strong>in</strong>g people and equipment. An example could be “bake, uncovered <strong>in</strong> preheated350 o F oven to an <strong>in</strong>ternal temperature <strong>of</strong> 165 o F for 15 seconds.”The HACCP team will f<strong>in</strong>d that many critical limits for your identified CCP’s have already been established.In some cases you’ll need more than one critical limit to control a particular hazard. For example, <strong>the</strong> typicalcritical limits for cooked beef patties are time/temperature, patty thickness, and conveyor speed. It isimportant that you identify all <strong>the</strong> critical limits for each <strong>of</strong> your products.Mak<strong>in</strong>g sure each Critical Control Po<strong>in</strong>t has critical limits is <strong>the</strong> responsibility <strong>of</strong> each establishment. The HACCPteam may want to get help from outside HACCP experts when establish<strong>in</strong>g critical limits. Remember that <strong>the</strong>critical limits must be able to ma<strong>in</strong>ta<strong>in</strong> control over <strong>the</strong> food safety hazard. Once <strong>the</strong> team has identified all <strong>the</strong>limits, enter <strong>the</strong>m onto <strong>the</strong> Critical Limits form.Page 45 <strong>of</strong> 146<strong>Meet<strong>in</strong>g</strong> <strong>the</strong> <strong>Requirements</strong> <strong>of</strong> <strong>the</strong> <strong>FDA</strong> <strong>Food</strong> <strong>Code</strong> <strong>Variance</strong> <strong>in</strong> Relation to Specialized Meat and Poultry Process<strong>in</strong>g Methods
Work<strong>in</strong>g with <strong>the</strong> “Critical Limits” FormFor each CCP <strong>the</strong> Example Facility has a separate page <strong>of</strong> critical limits.1. Under <strong>the</strong> “Limit” head<strong>in</strong>g. The Example Facility noted an <strong>in</strong>ternal temperature <strong>of</strong> 165 o F for 15 seconds as<strong>the</strong> established critical limit. They <strong>the</strong>n decided that <strong>the</strong> preventive measure <strong>of</strong> cook<strong>in</strong>g at 190 o F oventemperature for 3 hours would satisfy <strong>the</strong> critical limit.2. Under <strong>the</strong> “Source” Head<strong>in</strong>g. The Example Facility’s first source is regulatory and scientific. They decided totake <strong>the</strong> established regulatory limits and use <strong>the</strong>m, but <strong>the</strong>n <strong>the</strong>y also sent out samples <strong>of</strong> <strong>the</strong>ir f<strong>in</strong>ishedproduct to be scientifically analyzed. The results <strong>of</strong> <strong>the</strong> lab tests confirmed that <strong>the</strong>ir critical limits wereenough.[The source is <strong>the</strong> “evidence” that backs up your critical limits. The source provides that <strong>the</strong> critical limitsyou cite will effectively control <strong>the</strong> food safety hazards. Sources for critical limits can be scientific,regulatory or historical. The HACCP team has to f<strong>in</strong>d at least one source for each <strong>of</strong> your critical limits, butyou can always put more if you want.]When determ<strong>in</strong><strong>in</strong>g your critical limits make sure you file your support<strong>in</strong>gdocumentation with your HACCP plan. This documentation will help validate that <strong>the</strong>limits have been properly established. These could be th<strong>in</strong>gs such as letters fromoutside HACCP experts, or scientific reports, or lab test results. By hold<strong>in</strong>g onto <strong>the</strong>sePage 46 <strong>of</strong> 146<strong>Meet<strong>in</strong>g</strong> <strong>the</strong> <strong>Requirements</strong> <strong>of</strong> <strong>the</strong> <strong>FDA</strong> <strong>Food</strong> <strong>Code</strong> <strong>Variance</strong> <strong>in</strong> Relation to Specialized Meat and Poultry Process<strong>in</strong>g Methods
support<strong>in</strong>g documents you also provide verification material when needed.Page 47 <strong>of</strong> 146<strong>Meet<strong>in</strong>g</strong> <strong>the</strong> <strong>Requirements</strong> <strong>of</strong> <strong>the</strong> <strong>FDA</strong> <strong>Food</strong> <strong>Code</strong> <strong>Variance</strong> <strong>in</strong> Relation to Specialized Meat and Poultry Process<strong>in</strong>g Methods
Page 48 <strong>of</strong> 146<strong>Meet<strong>in</strong>g</strong> <strong>the</strong> <strong>Requirements</strong> <strong>of</strong> <strong>the</strong> <strong>FDA</strong> <strong>Food</strong> <strong>Code</strong> <strong>Variance</strong> <strong>in</strong> Relation to Specialized Meat and Poultry Process<strong>in</strong>g Methods
Pr<strong>in</strong>ciple 4: Establish Monitor<strong>in</strong>g ProceduresMonitor<strong>in</strong>g <strong>in</strong>volves a series <strong>of</strong> observations and/or measurements that are used to make sure a CCP is undercontrol. The HACCP team can th<strong>in</strong>k <strong>of</strong> monitor<strong>in</strong>g activities as <strong>the</strong> checks-and-balances for each CCP. Whensomeone monitors, <strong>the</strong>y are “check<strong>in</strong>g to see” that <strong>the</strong> critical limits are be<strong>in</strong>g met.What are <strong>the</strong> 3 th<strong>in</strong>gs monitor<strong>in</strong>g can do for you?Shows you when a deviation from a critical limit has happened. For example, an employee tests <strong>the</strong>temperature <strong>of</strong> some beef patties and discovers that <strong>the</strong> <strong>in</strong>ternal temperature has gone above <strong>the</strong>established critical limit <strong>of</strong> 40 o F. If not caught here, this would be a potentially serious health risk toconsumers.Helps you identify trends <strong>in</strong> your process that will allow you to predict a loss <strong>of</strong> control at a CCP. Forexample, a facility may monitor <strong>the</strong> temperature <strong>of</strong> a cold storage area at 6 a.m., 8 a.m., and 10 a.m. Eachtime, <strong>the</strong> temperature is with<strong>in</strong> acceptable limits, but it is steadily climb<strong>in</strong>g toward <strong>the</strong> high end <strong>of</strong> <strong>the</strong>range. This <strong>in</strong>formation po<strong>in</strong>ts towards a trend, and <strong>the</strong> facility should take action to prevent <strong>the</strong>temperature from exceed<strong>in</strong>g <strong>the</strong> critical limits.Produces written records for use <strong>in</strong> future HACCP plan verification steps. Written monitor<strong>in</strong>g records willprove very valuable to your operation, should a serious problem along <strong>the</strong> production l<strong>in</strong>e occur. Therecords you keep prove that your company has established and carried out effective monitor<strong>in</strong>g techniques.Monitor<strong>in</strong>g procedures can be thought <strong>of</strong> as cont<strong>in</strong>uous or non-cont<strong>in</strong>uous.• Cont<strong>in</strong>uous monitor<strong>in</strong>g is <strong>the</strong> constant monitor<strong>in</strong>g <strong>of</strong> a critical control po<strong>in</strong>t.• Non-cont<strong>in</strong>uous monitor<strong>in</strong>g is <strong>the</strong> scheduled monitor<strong>in</strong>g <strong>of</strong> a critical control po<strong>in</strong>t.Cont<strong>in</strong>uous monitor<strong>in</strong>g is always preferred when feasible. Cont<strong>in</strong>uous monitor<strong>in</strong>g at a CCP is usually done with built<strong>in</strong>measur<strong>in</strong>g equipment, such as a record<strong>in</strong>g <strong>the</strong>rmometer used at a cook<strong>in</strong>g step. This type <strong>of</strong> monitor<strong>in</strong>g ispreferred because it yields a permanent record. To make sure <strong>the</strong>se activities stay accurate, you need to regularlycheck <strong>the</strong> monitor<strong>in</strong>g equipment to make sure that it is calibrated correctly.If cont<strong>in</strong>uous monitor<strong>in</strong>g isn’t feasible for your CCP <strong>the</strong>n <strong>the</strong> HACCP team will need to establish non-cont<strong>in</strong>uousmonitor<strong>in</strong>g procedures. Non-cont<strong>in</strong>uous doesn’t mean random. The team should decide <strong>in</strong> <strong>the</strong> development phasewhat <strong>the</strong> monitor<strong>in</strong>g schedule should be. When you use non-cont<strong>in</strong>uous monitor<strong>in</strong>g, make sure that it’s scheduled<strong>of</strong>ten enough to keep <strong>the</strong> food safety hazards under control. Expert advice from people with knowledge <strong>of</strong> practicalstatistics and statistical process control will be important <strong>in</strong> mak<strong>in</strong>g your decisions. Types <strong>of</strong> non-cont<strong>in</strong>uousmonitor<strong>in</strong>g procedures <strong>in</strong>clude visual exam<strong>in</strong>ations, monitor<strong>in</strong>g <strong>in</strong>gredient specifications, measurements <strong>of</strong> pH orwater activity (Aw), tak<strong>in</strong>g product temperatures, etc.Who’s Responsible?Make sure to assign a specific person to be responsible for <strong>the</strong> monitor<strong>in</strong>g <strong>of</strong> a CCP. The Example Facility has aPage 49 <strong>of</strong> 146<strong>Meet<strong>in</strong>g</strong> <strong>the</strong> <strong>Requirements</strong> <strong>of</strong> <strong>the</strong> <strong>FDA</strong> <strong>Food</strong> <strong>Code</strong> <strong>Variance</strong> <strong>in</strong> Relation to Specialized Meat and Poultry Process<strong>in</strong>g Methods
designated shift leader/cook who is responsible for monitor<strong>in</strong>g <strong>the</strong> cook<strong>in</strong>g CCP. The person who actually does <strong>the</strong>monitor<strong>in</strong>g must be <strong>the</strong> person who signs and dates all <strong>the</strong> records at <strong>the</strong> time <strong>of</strong> monitor<strong>in</strong>g.Monitor<strong>in</strong>g will be most effective when:• The HACCP plan clearly identifies <strong>the</strong> employee(s) responsible for monitor<strong>in</strong>g.• Employees are tra<strong>in</strong>ed <strong>in</strong> <strong>the</strong> proper test<strong>in</strong>g procedures, <strong>the</strong> established critical limits, <strong>the</strong> methods <strong>of</strong>record<strong>in</strong>g monitor<strong>in</strong>g results, and <strong>the</strong> actions to be taken when critical limits are exceeded.• Employee(s) understand <strong>the</strong> purpose and importance <strong>of</strong> monitor<strong>in</strong>g.The last step <strong>in</strong> establish<strong>in</strong>g your monitor<strong>in</strong>g procedures is to develop <strong>the</strong> Monitor<strong>in</strong>g Log(s) where <strong>the</strong> monitor<strong>in</strong>gperson will record <strong>the</strong> date for each CCP. Due to <strong>the</strong> variety <strong>of</strong> monitor<strong>in</strong>g procedures, <strong>the</strong> HACCP team may needto developed different logs to record <strong>the</strong> monitor<strong>in</strong>g data at different CCP’s. When your HACCP system is up andrunn<strong>in</strong>g, you will use <strong>the</strong>se logs to track <strong>the</strong> day-to-day HACCP activities. Sample logs are provided <strong>in</strong> <strong>the</strong> Appendix.Work<strong>in</strong>g with <strong>the</strong> “Monitor<strong>in</strong>g Procedures” FormThe form that is shown as an example on <strong>the</strong> next page is to be used as a tool <strong>in</strong> <strong>the</strong> development <strong>of</strong> your HACCPplan. The <strong>in</strong>formation on this form is <strong>the</strong> “Who, What, When and How” <strong>of</strong> monitor<strong>in</strong>g.For <strong>the</strong> Example Facility:• The Who is <strong>the</strong> cook on duty.• The What is <strong>the</strong> temperature <strong>of</strong> <strong>the</strong> oven.• The When is non-cont<strong>in</strong>uously - every 60 m<strong>in</strong>utes, (+ 5 m<strong>in</strong>utes), and• The How is with <strong>the</strong> oven temperature gauge.The Example Store felt this type <strong>of</strong> non-cont<strong>in</strong>uous monitor<strong>in</strong>g would be effective because <strong>of</strong> <strong>the</strong> consistent heatenvironment <strong>of</strong> <strong>the</strong> oven. Their logic was that if <strong>the</strong> temperature taken at <strong>the</strong> beg<strong>in</strong>n<strong>in</strong>g and end <strong>of</strong> <strong>the</strong> cook<strong>in</strong>gcycle was <strong>the</strong> same, it could reasonably be assumed that it was okay for <strong>the</strong> whole cook<strong>in</strong>g cycle.Page 50 <strong>of</strong> 146<strong>Meet<strong>in</strong>g</strong> <strong>the</strong> <strong>Requirements</strong> <strong>of</strong> <strong>the</strong> <strong>FDA</strong> <strong>Food</strong> <strong>Code</strong> <strong>Variance</strong> <strong>in</strong> Relation to Specialized Meat and Poultry Process<strong>in</strong>g Methods
Page 51 <strong>of</strong> 146<strong>Meet<strong>in</strong>g</strong> <strong>the</strong> <strong>Requirements</strong> <strong>of</strong> <strong>the</strong> <strong>FDA</strong> <strong>Food</strong> <strong>Code</strong> <strong>Variance</strong> <strong>in</strong> Relation to Specialized Meat and Poultry Process<strong>in</strong>g Methods
Page 52 <strong>of</strong> 146<strong>Meet<strong>in</strong>g</strong> <strong>the</strong> <strong>Requirements</strong> <strong>of</strong> <strong>the</strong> <strong>FDA</strong> <strong>Food</strong> <strong>Code</strong> <strong>Variance</strong> <strong>in</strong> Relation to Specialized Meat and Poultry Process<strong>in</strong>g Methods
Page 53 <strong>of</strong> 146<strong>Meet<strong>in</strong>g</strong> <strong>the</strong> <strong>Requirements</strong> <strong>of</strong> <strong>the</strong> <strong>FDA</strong> <strong>Food</strong> <strong>Code</strong> <strong>Variance</strong> <strong>in</strong> Relation to Specialized Meat and Poultry Process<strong>in</strong>g Methods