View OnlineDownloaded by Centro de Química Orgánica "Lora Tamayo" on 27 January 2011Published on 25 January 2011 on http://pubs.rsc.org | doi:10.1039/C0GC00766H≥60% biodegradation by activated sludge microbial inoculate isrequired in a 28-day period. Full environmental biodegradationby microorganisms could yield completely non-toxic productsfrom potentially persistent and toxic solvents.The biodegradation study of ionic liquids is not easy. Firstly,they consist of large organic cations, such as imidazolium, pyridinium,pyrrolidinium, ammonium and phosphonium, amongothers, with alkyl chain substituents that alter the hydrophobicityof the compounds, and combined with a variety ofanions of different chemical nature, giving an enormous rangeof cation/anion combinations. This results in the potential forstructure-property control for specific tasks, which gives riseto specific ILs, so called functionalized ionic liquids. 12 Manydifferent functional groups have already been incorporated intoionic liquid cations and anions, e.g., alkenes, ethers, esters,alcohols, etc. Consequently, understanding the biodegradabilitymechanism of these functionalized ILs becomes more complicated.This difficulty is common to studies on the toxicityof ILs, as revealed by our group in previous works applyingseveral models. 13,14 The first attempts to examine the degradationpotential of 1-butyl-3-methylimidazolium (Bmim + ) based ILswith common anions (Br - ,BF 4- ,PF 6- , etc.) were carried outby the group of Scammells. 15–19 Using standard test protocols,they revealed that those ILs did not show a significant degree ofbiodegradation, with the exception of octyl-sulfate-containingILs. The efforts were then centred on the design of ILswith enhanced biodegradability – assisted by Boethling andcoworkers guidelines – for the synthesis of environmentallybenign solvents. 20 The results indicated that some structuralcharacteristics of ILs enhance or reduce the possibility foran enzymatic cleavage of ILs. Thus, it was demonstrated thatthe biodegradability depends on the length of the alkyl sidechain of the cationic head group. 15,21 The longer the alkyl sidechain (>C6) the better the biodegradability is. However, thiseffect does not seem to be endlessly valid due to an increasingtoxicity to microorganisms with increasing alkyl side chainlength. In this sense, ester functional groups were successfullyintroduced into the alkyl side chain of ILs to enhance thebiodegradability of imidazolium and pyridinium based ILs. 16,17Furthermore, the biodegradability of ILs has been demonstratedto be dependent on the structure of anion. 18 Biodegradableanions are usually organic species, such as octyl sulfates,acetate and naphthenic acids like 3-cylcohexylpropionate. 18,22For inorganic anions, the biodegradation efficiency 18 decreasedin the order PF 6- > BF 4- > Br - > Cl - . 6 The reported studiesindicated that, in general, common ILs are not readilybiodegradable. 23 The incorporation of other functional groups(hydroxyl, carboxyl, ether and nitrile) to alkyl chain of ILs didnot improve their biological degradation. 23 Wells and Coombe 24and other authors 25 extended the microbial degradation studyto ammonium and phosphonium compounds. In agreementwith other works, 15,21 no biodegradability was observed forcations with short chains (C £ 4), whereas for long alkylchains (C ≥ 12) a strong inhibitory effect on the activity ofinoculum was found due to IL toxicity. However, further studiesreported pyridinium-based ILs being effectively catabolised bymicrobial community in activated sludge. 23,25–31 In fact, fullmineralisation was achieved for specifically designed ILs asthose constituted by pyridinium rings with longer alkyl sidechains or hydroxyl groups combined with biodegradable anionsas saccharinate or octylsulfate. 29,30 So far, the reported studieshave been centered to determine the biodegradability of ILsunder aerobic conditions. Very recently, the primary anaerobicbiodegradability of imidazolium and pyridinium based ILswas investigated under nitrogen reducing conditions, obtainingeven worse results under denitrifying conditions compared tothose under aerobic ones. 32 In any case, the above mentionedstudies failed to systematically screen a single microorganism ormicrobial consortium responsible for biodegradation of ILs. Adifferent strategy in biodegradation studies of ILs was conductedby Kumar et al., 33 who demonstrated that the wastewatermicroorganism Pseudomonas putida was able to break downthe ionic liquid BmimBF 4 after 15 days of incubation, evenwhen this compound was indicated to be recalcitrant in Sturmand closed-bottle test assays. 15,16 Other microorganisms such assoil Corynebacteria 34 or aquatic Daphnia magna 35 were able tobiodegrade pyridinium-based ILs, but not those ILs constitutedby imidazolium cations. In this sense, it is necessary to furtherinvestigate which type of microorganisms are adaptable to ILsand which are responsible for degradation, also to suggest initialguidelines for the treatment of IL waste by using the existingaerobic and anaerobic wastewater treatment facilities.In the present investigation, we propose a microorganism(S. paucimobilis bacterium), so far not investigated in thisfield, to examine the biodegradability of a wide sample (37)of commercial ILs. The work was performed with the followingnovelties in the field of IL biodegradability:a) The biodegradation of ILs was monitored by the productionof carbon dioxide using an indirect impedance technique.For the first time, we apply to IL biodegradability determinationa methodology based on the measurement of the evolution ofpotassium hydroxide solution as the biodegradation of ionicliquid takes place. The method, based on trapping the carbondioxide produced by the microbial metabolism, is simple andit is well proven in other areas, such as the quality controlof certain foods 36,37 or manufacturing industries, 38,39 to detectmicrobial contaminations. The use of modern commercial systemsmakes an automatic method possible for the simultaneousand reliable measurements of different samples during the longtime necessary to biodegrade chemicals products (in this work28 days). 40–43b) Experiments are carried out with controlled inocula of theS. paucimobilis mesophilic/thermophilic bacterium, which wasisolated from cinematographic films and identified in a previouswork. 44 This bacterium has been proven to be very efficientat biodegrading different organic chemicals such us PAHsuntil five condensed benzene rings. 45,46 Due to the efficiency ofS. paucimobilis to biodegrade aromatic compounds and its resistanceto environmental extreme conditions, we have consideredusing this bacterium to evaluate the biodegradation of ILs. Thetemperature of biodegradation assays has been optimized inorder to enhance the bacterium metabolism effectiveness. Onthe other hand, the system supports a significant concentrationofILs,between0.5and3gL -1 , where it usually operates ataround 20–50 mg L -1 . Both temperature and concentrationlevels, very high compared to the study so far, could contributeto accelerating a possible biological process of wastewatertreatment with ILs.<strong>Green</strong> Chem. This journal is © The Royal Society of <strong>Chemistry</strong> 2011
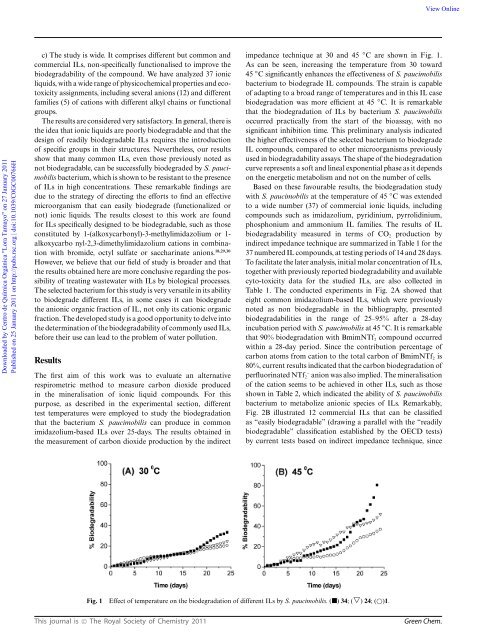
View OnlineDownloaded by Centro de Química Orgánica "Lora Tamayo" on 27 January 2011Published on 25 January 2011 on http://pubs.rsc.org | doi:10.1039/C0GC00766Hc) The study is wide. It comprises different but common andcommercial ILs, non-specifically functionalised to improve thebiodegradability of the compound. We have analyzed 37 ionicliquids, with a wide range of physicochemical properties and ecotoxicityassignments, including several anions (12) and differentfamilies (5) of cations with different alkyl chains or functionalgroups.The results are considered very satisfactory. In general, there isthe idea that ionic liquids are poorly biodegradable and that thedesign of readily biodegradable ILs requires the introductionof specific groups in their structures. Nevertheless, our resultsshow that many common ILs, even those previously noted asnot biodegradable, can be successfully biodegraded by S. paucimobilisbacterium, which is shown to be resistant to the presenceof ILs in high concentrations. These remarkable findings aredue to the strategy of directing the efforts to find an effectivemicroorganism that can easily biodegrade (functionalized ornot) ionic liquids. The results closest to this work are foundfor ILs specifically designed to be biodegradable, such as thoseconstituted by 1-(alkoxycarbonyl)-3-methylimidazolium or 1-alkoxycarbo nyl-2,3-dimethylimidazolium cations in combinationwith bromide, octyl sulfate or saccharinate anions. 18,29,30However, we believe that our field of study is broader and thatthe results obtained here are more conclusive regarding the possibilityof treating wastewater with ILs by biological processes.The selected bacterium for this study is very versatile in its abilityto biodegrade different ILs, in some cases it can biodegradethe anionic organic fraction of IL, not only its cationic organicfraction. The developed study is a good opportunity to delve intothe determination of the biodegradability of commonly used ILs,before their use can lead to the problem of water pollution.ResultsThe first aim of this work was to evaluate an alternativerespirometric method to measure carbon dioxide producedin the mineralisation of ionic liquid compounds. For thispurpose, as described in the experimental section, differenttest temperatures were employed to study the biodegradationthat the bacterium S. paucimobilis can produce in commonimidazolium-based ILs over 25-days. The results obtained inthe measurement of carbon dioxide production by the indirectimpedance technique at 30 and 45 ◦ C are shown in Fig. 1.As can be seen, increasing the temperature from 30 toward45 ◦ C significantly enhances the effectiveness of S. paucimobilisbacterium to biodegrade IL compounds. The strain is capableof adapting to a broad range of temperatures and in this IL casebiodegradation was more efficient at 45 ◦ C. It is remarkablethat the biodegradation of ILs by bacterium S. paucimobilisoccurred practically from the start of the bioassay, with nosignificant inhibition time. This preliminary analysis indicatedthe higher effectiveness of the selected bacterium to biodegradeIL compounds, compared to other microorganisms previouslyused in biodegradability assays. The shape of the biodegradationcurve represents a soft and lineal exponential phase as it dependson the energetic metabolism and not on the number of cells.Based on these favourable results, the biodegradation studywith S. paucimobilis at the temperature of 45 ◦ C was extendedto a wide number (37) of commercial ionic liquids, includingcompounds such as imidazolium, pyridinium, pyrrolidinium,phosphonium and ammonium IL families. The results of ILbiodegradability measured in terms of CO 2 production byindirect impedance technique are summarized in Table 1 for the37 numbered IL compounds, at testing periods of 14 and 28 days.To facilitate the later analysis, initial molar concentration of ILs,together with previously reported biodegradability and availablecyto-toxicity data for the studied ILs, are also collected inTable 1. The conducted experiments in Fig. 2A showed thateight common imidazolium-based ILs, which were previouslynoted as non biodegradable in the bibliography, presentedbiodegradabilities in the range of 25–95% after a 28-dayincubation period with S. paucimobilis at 45 ◦ C. It is remarkablethat 90% biodegradation with BmimNTf 2 compound occurredwithin a 28-day period. Since the contribution percentage ofcarbon atoms from cation to the total carbon of BmimNTf 2 is80%, current results indicated that the carbon biodegradation ofperfluorinated NTf 2- anion was also implied. The mineralisationofthecationseemstobeachievedinotherILs,suchasthoseshown in Table 2, which indicated the ability of S. paucimobilisbacterium to metabolize anionic species of ILs. Remarkably,Fig. 2B illustrated 12 commercial ILs that can be classifiedas “easily biodegradable” (drawing a parallel with the “readilybiodegradable” classification established by the OECD tests)by current tests based on indirect impedance technique, sinceFig. 1Effect of temperature on the biodegradation of different ILs by S. paucimobilis. () 34;(▽) 24;()1.This journal is © The Royal Society of <strong>Chemistry</strong> 2011<strong>Green</strong> Chem.