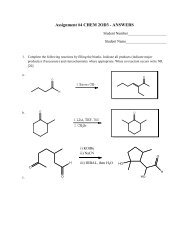
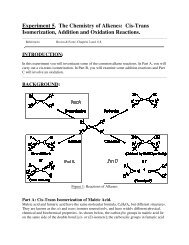
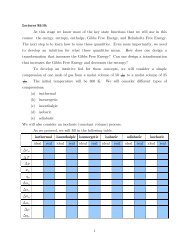
Tutorial 5
Tutorial 5
Tutorial 5
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
or ln (0.8310) = -E a × (333 - 298)8.314 J mol -1 K -1 298 × 333or - 0.1851 = -E a × 358.314 298 × 333Isolate for E a , E a = 0.1851 × 8.314 × 298 × 33335= 4363 J mol -1 or 4.36 kJ mol -1(b)If the rate constant at the cooler temperature is 2.30 × 10 9 M −1 s −1 , then we mayuse the data either from the 25°C or 60 o C experiment together with thecalculated E a to obtain the value of the temperature.Thus ln (5.9 × 10 9 ) = ln A - 4.36 kJ . 1 --------(1)R 298and ln (2.30 × 10 9 ) = ln A - 4.36 kJ . 1 --------(2)R T 2Take equation (2) -(1)ln(5.9 x 10 9 ) - ln (2.30 × 10 9 ) = - 4.36 × 10 3 J mol -1 . ( 1 - 1 ) . 18.314 J mol -1 K -1 298 T 2 Kor 0.9420 = 4.36 × 10 3 (3.356 × 10 −3 - 1 )8.314 T 25.152 × 10 −3 = 1 or T 2 = 194 K, and 194 – 273 = −79 °CT 27. Biological reactions nearly always occur in the presence of enzymes, which are verypowerful catalysts. For example, the enzyme catalase that acts on peroxides reduces theactivation energy of peroxide decomposition from 72 kJ/mol (in the uncatalyzedreaction) to 28 kJ/mol (in the catalyzed reaction) at 298 K. What is the increase in kwhen the reaction is catalyzed? (Assume the frequency factor A remains constant).SOLUTION:Recall we can have the Arrhenius equation in the formln k = ln A - E aRTSo if we write this equation for both sets of experimental conditions,ln k 1 = ln A − E a1 (eq. 1) and ln k 2 = ln A − E a2 (eq.2)RTRT4