<strong>Haematologica</strong> 2000; 85(the Platelet <strong>ADP</strong> Receptors Supplement):53-57HUMAN ECTO-<strong>ADP</strong>ASE/CD39: THROMBOREGULATIONVIA A NOVEL PATHWAYAARON J. MARCUS, M. JOHAN BROEKMAN, JOAN H.F. DROSOPOULOS, NAZIBA ISLAM, RICHARD B. GAYLE III,*DAVID J. PINSKY,° CHARLES R. MALISZEWSKI*VA New York Harbor Healthcare System and Weill Medical College of Cornell University, New York, NY; *Immunex Corp.,Seattle, WA; °Columbia University, College of Physicians and Surgeons, New York, NY, USAABSTRACTVascular injury in coronary, carotid, and peripheralarteries evokes local platelet activation, recruitmentand thrombotic occlusion. Platelets are unresponsiveto agonists in the presence of endothelialcells, even in the absence of eicosanoids and nitricoxide. We have characterized endothelial cellCD39/ecto-<strong>ADP</strong>ase as the prime thromboregulator.CD39 rapidly and preferentially metabolizes <strong>ADP</strong>released from activated platelets, thereby abolishingaggregation and recruitment. Our recombinant,soluble form of human CD39, solCD39, a glycosylated66 kD protein, possesses the same enzymaticand biological properties as full-length CD39. Sol-CD39 blocked <strong>ADP</strong>-induced human platelet aggregationin vitro, and inhibited collagen- and TRAPinducedplatelet reactivity. SolCD39 was studied invivo in a murine stroke model driven by excessiveplatelet recruitment. In CD39 +/+ mice, solCD39completely abolished <strong>ADP</strong>-induced platelet aggregation,and strongly inhibited collagen- and arachidonate-inducedaggregates ex vivo. When administeredprior to transient intraluminal right middlecerebral artery occlusion, solCD39 reduced ipsilateralfibrin deposition, decreased 111 In-platelet deposition,and increased post-ischemic blood flow twofoldat 24 hr. These results were better than thoseobtained with aspirin. CD39 –/– mice, generated bydeleting exons 4-6 (apyrase conserved regions 2-4),had normal phenotypes, hematologic profiles andbleeding times, but exhibited a decrease in postischemicperfusion and an increase in cerebralinfarct volume as compared to genotypic CD39 +/+controls. CD39 –/– mice, reconstituted with sol-CD39, had increased post-ischemic flow and wererescued from cerebral injury. We conclude that sol-CD39 has potential as a novel therapeutic agentfor thrombotic diatheses.© 2000, Ferrata Storti FoundationCorrespondence: Aaron J. Marcus, M.D./151B, Chief, Hematology-Medical Oncology, VA New York Harbor Health Care System, New York,NY, USA. E-mail: ajmarcus@med.cornell.edu, mjbroek@med.cornell.eduIntroductionCell-cell interactions and cell-vessel wall interactionsare of critical importance for hemostasis.Many of these interactions occur via transcellularmetabolism, a locution that indicates reciprocal orcollaborative metabolism of signaling molecules bydifferent cells. This is particularly pertinent in thecase of endothelial cells and platelets. We currentlybelieve that endothelial cells downregulate plateletreactivity via at least three different pathways: a cellassociatedaspirin-insensitive nucleotidase, 1 and twoindependent short-lived fluid-phase signaling systems- eicosanoids such as prostacyclin (PGI 2 ); 2 andthe nitric oxide (NO) system. 3 In 1991, we <strong>doc</strong>umentedthat platelet reactivity remained inhibited byendothelial cells under experimental conditionswhich rendered NO ineffective, even when both celltypes were aspirin-treated, to delete PGI 2 from thesystem. Using biochemical and functional measurementtechniques, we determined that aspirin-treatedhuman umbilical vein endothelial cells (HUVEC)inhibited platelet function in vitro largely via metabolismof <strong>ADP</strong> from the releasate generated by plateletsactivated by a variety of agonists. This metabolism of<strong>ADP</strong> resulted in loss of platelet activation, release,recruitment, and aggregation. 1 This paradigm ofplatelet inhibition is unique in that it does not interferewith platelet function except for removal of thesoluble phase agonist responsible for excessiveplatelet activation and recruitment. Such conditionswould otherwise promote thrombosis. Our data suggestthat enhancing the activity of this pathway hasa strong antithrombotic action, without significantlyreducing the hemostatic effectiveness of platelets.Identification of CD39 as the endothelialcell ecto-<strong>ADP</strong>ase responsible forInhibition of platelet functionWe initially identified the ability of endothelial cellsto inhibit platelet reactivity via metabolism of <strong>ADP</strong>,rather than via eicosanoids or NO: aspirin-treatedHUVEC were incubated with radio-labeled <strong>ADP</strong>.Under these conditions, no PGI 2 was formed, andany NO generated rapidly decayed or was blocked byaddition of purified oxyhemoglobin. Radio-TLC wasemployed to separate and identify <strong>ADP</strong> and itsmetabolites (Figure 1). Cell-free supernatant fromthe incubation of HUVEC with <strong>ADP</strong> was transferredto aggregometry cuvettes containing platelet-rich<strong>Haematologica</strong> vol. 85 (the Platelet <strong>ADP</strong> Receptors supplement), June 2000
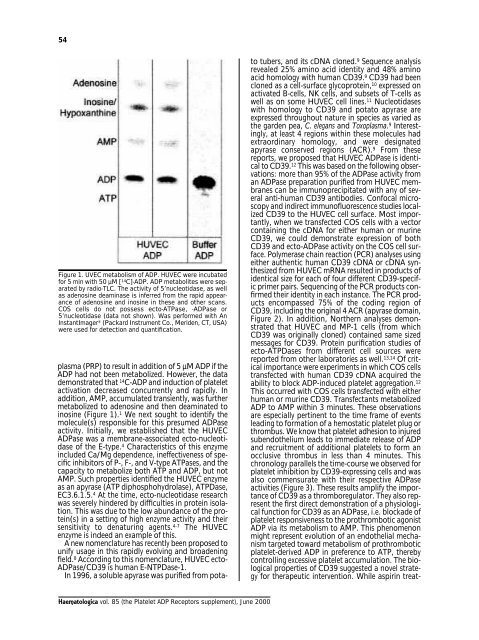
54Figure 1. UVEC metabolism of <strong>ADP</strong>. HUVEC were incubatedfor 5 min with 50 µM [ 14 C]-<strong>ADP</strong>. <strong>ADP</strong> metabolites were separatedby radio-TLC. The activity of 5’nucleotidase, as wellas adenosine deaminase is inferred from the rapid appearanceof adenosine and inosine in these and other scans.COS cells do not possess ecto-ATPase, -<strong>ADP</strong>ase or5’nucleotidase (data not shown). Was performed with AnInstantImager ® (Packard Instrument Co., Meriden, CT, USA)were used for detection and quantification.plasma (PRP) to result in addition of 5 µM <strong>ADP</strong> if the<strong>ADP</strong> had not been metabolized. However, the datademonstrated that 14 C-<strong>ADP</strong> and induction of plateletactivation decreased concurrently and rapidly. Inaddition, AMP, accumulated transiently, was furthermetabolized to adenosine and then deaminated toinosine (Figure 1). 1 We next sought to identify themolecule(s) responsible for this presumed <strong>ADP</strong>aseactivity. Initially, we established that the HUVEC<strong>ADP</strong>ase was a membrane-associated ecto-nucleotidaseof the E-type. 4 Characteristics of this enzymeincluded Ca/Mg dependence, ineffectiveness of specificinhibitors of P-, F-, and V-type ATPases, and thecapacity to metabolize both ATP and <strong>ADP</strong>, but notAMP. Such properties identified the HUVEC enzymeas an apyrase (ATP diphosphohydrolase), ATPDase,EC3.6.1.5. 4 At the time, ecto-nucleotidase researchwas severely hindered by difficulties in protein isolation.This was due to the low abundance of the protein(s)in a setting of high enzyme activity and theirsensitivity to denaturing agents. 4-7 The HUVECenzyme is indeed an example of this.A new nomenclature has recently been proposed tounify usage in this rapidly evolving and broadeningfield. 8 According to this nomenclature, HUVEC ecto-<strong>ADP</strong>ase/CD39 is human E-NTPDase-1.In 1996, a soluble apyrase was purified from potatotubers, and its cDNA cloned. 9 Sequence analysisrevealed 25% amino acid identity and 48% aminoacid homology with human CD39. 9 CD39 had beencloned as a cell-surface glycoprotein, 10 expressed onactivated B-cells, NK cells, and subsets of T-cells aswell as on some HUVEC cell lines. 11 Nucleotidaseswith homology to CD39 and potato apyrase areexpressed throughout nature in species as varied asthe garden pea, C. elegans and Toxoplasma. 9 Interestingly,at least 4 regions within these molecules hadextraordinary homology, and were designatedapyrase conserved regions (ACR). 9 From thesereports, we proposed that HUVEC <strong>ADP</strong>ase is identicalto CD39. 12 This was based on the following observations:more than 95% of the <strong>ADP</strong>ase activity froman <strong>ADP</strong>ase preparation purified from HUVEC membranescan be immunoprecipitated with any of severalanti-human CD39 antibodies. Confocal microscopyand indirect immunofluorescence studies localizedCD39 to the HUVEC cell surface. Most importantly,when we transfected COS cells with a vectorcontaining the cDNA for either human or murineCD39, we could demonstrate expression of bothCD39 and ecto-<strong>ADP</strong>ase activity on the COS cell surface.Polymerase chain reaction (PCR) analyses usingeither authentic human CD39 cDNA or cDNA synthesizedfrom HUVEC mRNA resulted in products ofidentical size for each of four different CD39-specificprimer pairs. Sequencing of the PCR products confirmedtheir identity in each instance. The PCR productsencompassed 75% of the coding region ofCD39, including the original 4 ACR (apyrase domain,Figure 2). In addition, Northern analyses demonstratedthat HUVEC and MP-1 cells (from whichCD39 was originally cloned) contained same sizedmessages for CD39. Protein purification studies ofecto-ATPDases from different cell sources werereported from other laboratories as well. 13,14 Of criticalimportance were experiments in which COS cellstransfected with human CD39 cDNA acquired theability to block <strong>ADP</strong>-induced platelet aggregation. 12This occurred with COS cells transfected with eitherhuman or murine CD39. Transfectants metabolized<strong>ADP</strong> to AMP within 3 minutes. These observationsare especially pertinent to the time frame of eventsleading to formation of a hemostatic platelet plug orthrombus. We know that platelet adhesion to injuredsubendothelium leads to immediate release of <strong>ADP</strong>and recruitment of additional platelets to form anocclusive thrombus in less than 4 minutes. Thischronology parallels the time-course we observed forplatelet inhibition by CD39-expressing cells and wasalso commensurate with their respective <strong>ADP</strong>aseactivities (Figure 3). These results amplify the importanceof CD39 as a thromboregulator. They also representthe first direct demonstration of a physiologicalfunction for CD39 as an <strong>ADP</strong>ase, i.e. blockade ofplatelet responsiveness to the prothrombotic agonist<strong>ADP</strong> via its metabolism to AMP. This phenomenonmight represent evolution of an endothelial mechanismtargeted toward metabolism of prothromboticplatelet-derived <strong>ADP</strong> in preference to ATP, therebycontrolling excessive platelet accumulation. The biologicalproperties of CD39 suggested a novel strategyfor therapeutic intervention. While aspirin treat-<strong>Haematologica</strong> vol. 85 (the Platelet <strong>ADP</strong> Receptors supplement), June 2000
- Page 1 and 2:
Haematologicaestablished in 1920 ed
- Page 3 and 4:
Editorial policy, subscriptions and
- Page 5 and 6:
to fit the width of a single column
- Page 8 and 9:
The Chairmen of the Congress would
- Page 10 and 11:
Haematologica 2000; 85 (The Platele
- Page 12 and 13: Haematologica 2000; 85(the Platelet
- Page 14 and 15: 5myocardial infarction …, the rat
- Page 16 and 17: 7Table 2. Inhibitors of platelet ag
- Page 18 and 19: 9AcknowledgmentsThis work was suppo
- Page 20 and 21: Haematologica 2000; 85(the Platelet
- Page 22 and 23: 13We labeled 2-methylthio ADP with
- Page 24 and 25: Haematologica 2000; 85(the Platelet
- Page 26 and 27: 17cell lines (Jurkat, MOLT-4, JM-1,
- Page 28 and 29: 19References1. Webb TE, Simon J, Kr
- Page 30 and 31: 21Biochem Biophys Res Comm 1989; 16
- Page 32 and 33: 23Figure 1. Overview of human plate
- Page 34 and 35: 25tion with nitric oxide calcium mo
- Page 36 and 37: Haematologica 2000; 85(the Platelet
- Page 38 and 39: 29that signaling through G q is ess
- Page 40 and 41: 312030-4.42. Fagura MS, Dainty IA,
- Page 42 and 43: 33an important physiologic platelet
- Page 44 and 45: 352. Cattaneo M, Gachet C. ADP rece
- Page 46 and 47: Haematologica 2000; 85(the Platelet
- Page 48 and 49: 39GPIbβ for one allele leading to
- Page 50 and 51: 41Hereditary X-linked thrombocytope
- Page 52 and 53: 43Beta3-integrin-deficient mice are
- Page 54 and 55: 45drome type 1 gene. J Biol Chem 20
- Page 56 and 57: 47induced by ADP. 10,15,16 Antagoni
- Page 58 and 59: 49Figure 3. Electron micrograph sho
- Page 60 and 61: 51such as AR-C69931 (a therapeutica
- Page 64 and 65: 55Figure 2. Domain structure of ect
- Page 66 and 67: 57CD39 prevented inhibition of plat
- Page 68 and 69: 59as inhibitors of adenylate cyclas
- Page 70 and 71: 61generation of genetically-modifie
- Page 72 and 73: 63niques. 68,69,70,71 However, the
- Page 74 and 75: 65macol 1997; 120:131P.61. Jarvis G
- Page 76 and 77: 67the P2T subtype with the (then) u
- Page 78 and 79: 69co-activation of both the Gi and
- Page 80 and 81: 71logic tools in defining the P2 re
- Page 82 and 83: Haematologica 2000; 85(the Platelet
- Page 84 and 85: 75tic cell lines, have not been suc
- Page 86 and 87: 77in rat platelets, Br J Haematol 1
- Page 88 and 89: 79Table 1. STIMS.Table 3. STAI.Pati
- Page 90 and 91: Haematologica 2000; 85(the Platelet
- Page 92: The Platelet ADP ReceptorsOral comm
- Page 95 and 96: 86ADP INDUCES PARTIAL PLATELET AGGR
- Page 97 and 98: 88response requiring concomitant ac
- Page 99 and 100: 90two other MAP kinases, ERK1 and E
- Page 101 and 102: 92each GPIIb/IIIa antagonist produc
- Page 103 and 104: 94POSTERSINVOLVEMENT OF THE P2CYC B
- Page 105 and 106: 96this parameter constrained to uni
- Page 107 and 108: 98when apyrase is added after the a
- Page 109 and 110: 100gometer, whereas it only causes
- Page 111: Direttore responsabile: Prof. Edoar