Template for Electronic Submission to ACS Journals - BioTechnologia
Template for Electronic Submission to ACS Journals - BioTechnologia
Template for Electronic Submission to ACS Journals - BioTechnologia
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
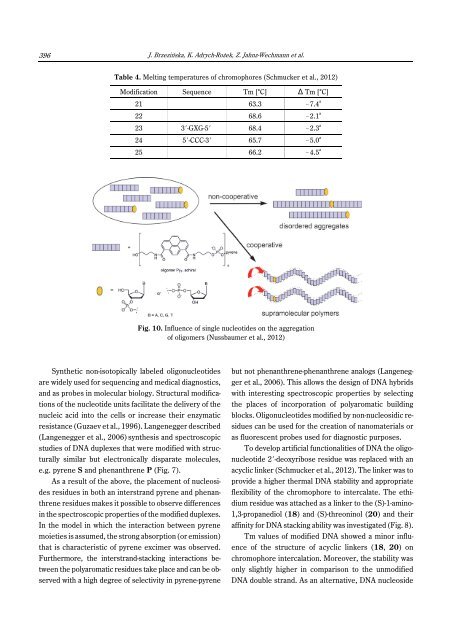
396J. Brzezińska, K. Adrych-Rożek, Z. Jahnz-Wechmann et al.Table 4. Melting temperatures of chromophores (Schmucker et al., 2012)Modification Sequence Tm [°C] ) Tm [°C]21 63.3 !7.4 a22 68.6 !2.1 a23 3N-GXG-5N 68.4 !2.3 a24 5N-CCC-3N 65.7 !5.0 a25 66.2 !4.5 aFig. 10. Influence of single nucleotides on the aggregationof oligomers (Nussbaumer et al., 2012)Synthetic non-iso<strong>to</strong>pically labeled oligonucleotidesare widely used <strong>for</strong> sequencing and medical diagnostics,and as probes in molecular biology. Structural modificationsof the nucleotide units facilitate the delivery of thenucleic acid in<strong>to</strong> the cells or increase their enzymaticresistance (Guzaev et al., 1996). Langenegger described(Langenegger et al., 2006) synthesis and spectroscopicstudies of DNA duplexes that were modified with structurallysimilar but electronically disparate molecules,e.g. pyrene S and phenanthrene P (Fig. 7).As a result of the above, the placement of nucleosidesresidues in both an interstrand pyrene and phenanthreneresidues makes it possible <strong>to</strong> observe differencesin the spectroscopic properties of the modified duplexes.In the model in which the interaction between pyrenemoieties is assumed, the strong absorption (or emission)that is characteristic of pyrene excimer was observed.Furthermore, the interstrand-stacking interactions betweenthe polyaromatic residues take place and can be observedwith a high degree of selectivity in pyrene-pyrenebut not phenanthrene-phenanthrene analogs (Langeneggeret al., 2006). This allows the design of DNA hybridswith interesting spectroscopic properties by selectingthe places of incorporation of polyaromatic buildingblocks. Oligonucleotides modified by non-nucleosidic residuescan be used <strong>for</strong> the creation of nanomaterials oras fluorescent probes used <strong>for</strong> diagnostic purposes.To develop artificial functionalities of DNA the oligonucleotide2N-deoxyribose residue was replaced with anacyclic linker (Schmucker et al., 2012). The linker was <strong>to</strong>provide a higher thermal DNA stability and appropriateflexibility of the chromophore <strong>to</strong> intercalate. The ethidiumresidue was attached as a linker <strong>to</strong> the (S)-1-amino-1,3-propanediol (18) and (S)-threoninol (20) and theiraffinity <strong>for</strong> DNA stacking ability was investigated (Fig. 8).Tm values of modified DNA showed a minor influenceof the structure of acyclic linkers (18, 20) onchromophore intercalation. Moreover, the stability wasonly slightly higher in comparison <strong>to</strong> the unmodifiedDNA double strand. As an alternative, DNA nucleoside