Synthesis of Tyrosinase Inhibitors: Designing Chalcones
Synthesis of Tyrosinase Inhibitors: Designing Chalcones
Synthesis of Tyrosinase Inhibitors: Designing Chalcones
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
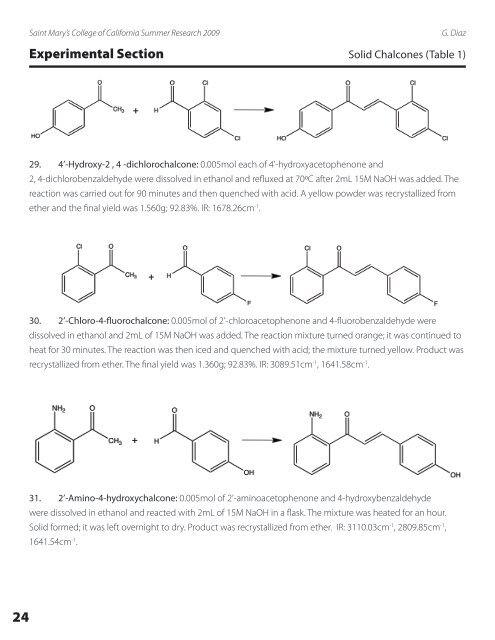
Saint Mary’s College <strong>of</strong> California Summer Research 2009G. DiazExperimental Section Solid <strong>Chalcones</strong> (Table 1)+29. 4’-Hydroxy-2 , 4 -dichlorochalcone: 0.005mol each <strong>of</strong> 4’-hydroxyacetophenone and2, 4-dichlorobenzaldehyde were dissolved in ethanol and refluxed at 70ºC after 2mL 15M NaOH was added. Thereaction was carried out for 90 minutes and then quenched with acid. A yellow powder was recrystallized fromether and the final yield was 1.560g; 92.83%. IR: 1678.26cm -1 .+30. 2’-Chloro-4-fluorochalcone: 0.005mol <strong>of</strong> 2’-chloroacetophenone and 4-fluorobenzaldehyde weredissolved in ethanol and 2mL <strong>of</strong> 15M NaOH was added. The reaction mixture turned orange; it was continued toheat for 30 minutes. The reaction was then iced and quenched with acid; the mixture turned yellow. Product wasrecrystallized from ether. The final yield was 1.360g; 92.83%. IR: 3089.51cm -1 , 1641.58cm -1 .+31. 2’-Amino-4-hydroxychalcone: 0.005mol <strong>of</strong> 2’-aminoacetophenone and 4-hydroxybenzaldehydewere dissolved in ethanol and reacted with 2mL <strong>of</strong> 15M NaOH in a flask. The mixture was heated for an hour.Solid formed; it was left overnight to dry. Product was recrystallized from ether. IR: 3110.03cm -1 , 2809.85cm -1 ,1641.54cm -1 .24