PG11 Anxiety Spectrum and Related Disorders - Devon Partnership ...
PG11 Anxiety Spectrum and Related Disorders - Devon Partnership ...
PG11 Anxiety Spectrum and Related Disorders - Devon Partnership ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
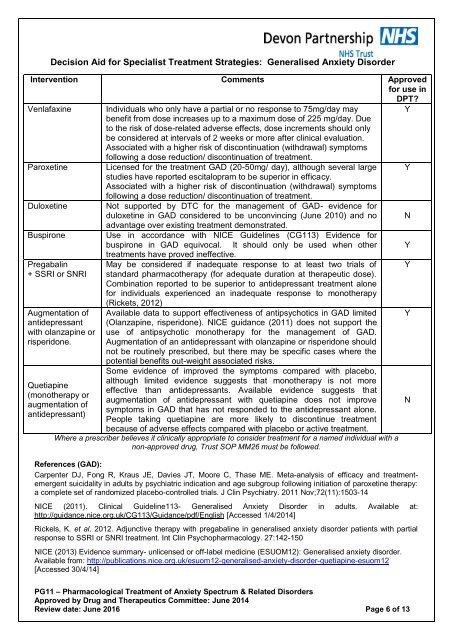
Decision Aid for Specialist Treatment Strategies: Generalised <strong>Anxiety</strong> DisorderIntervention Comments Approvedfor use inDPT?Venlafaxine Individuals who only have a partial or no response to 75mg/day mayYbenefit from dose increases up to a maximum dose of 225 mg/day. Dueto the risk of dose-related adverse effects, dose increments should onlybe considered at intervals of 2 weeks or more after clinical evaluation.Associated with a higher risk of discontinuation (withdrawal) symptomsfollowing a dose reduction/ discontinuation of treatment.Paroxetine Licensed for the treatment GAD (20-50mg/ day), although several large Ystudies have reported escitalopram to be superior in efficacy.Associated with a higher risk of discontinuation (withdrawal) symptomsfollowing a dose reduction/ discontinuation of treatment.Duloxetine Not supported by DTC for the management of GAD- evidence forduloxetine in GAD considered to be unconvincing (June 2010) <strong>and</strong> no Nadvantage over existing treatment demonstrated.Buspirone Use in accordance with NICE Guidelines (CG113) Evidence forbuspirone in GAD equivocal. It should only be used when other YPregabalin+ SSRI or SNRIAugmentation ofantidepressantwith olanzapine orrisperidone.Quetiapine(monotherapy oraugmentation ofantidepressant)treatments have proved ineffective.May be considered if inadequate response to at least two trials ofst<strong>and</strong>ard pharmacotherapy (for adequate duration at therapeutic dose).Combination reported to be superior to antidepressant treatment alonefor individuals experienced an inadequate response to monotherapy(Rickets, 2012)Available data to support effectiveness of antipsychotics in GAD limited(Olanzapine, risperidone). NICE guidance (2011) does not support theuse of antipsychotic monotherapy for the management of GAD.Augmentation of an antidepressant with olanzapine or risperidone shouldnot be routinely prescribed, but there may be specific cases where thepotential benefits out-weight associated risks.Some evidence of improved the symptoms compared with placebo,although limited evidence suggests that monotherapy is not moreeffective than antidepressants. Available evidence suggests thataugmentation of antidepressant with quetiapine does not improvesymptoms in GAD that has not responded to the antidepressant alone.People taking quetiapine are more likely to discontinue treatmentbecause of adverse effects compared with placebo or active treatment.Where a prescriber believes it clinically appropriate to consider treatment for a named individual with anon-approved drug, Trust SOP MM26 must be followed.References (GAD):Carpenter DJ, Fong R, Kraus JE, Davies JT, Moore C, Thase ME. Meta-analysis of efficacy <strong>and</strong> treatmentemergentsuicidality in adults by psychiatric indication <strong>and</strong> age subgroup following initiation of paroxetine therapy:a complete set of r<strong>and</strong>omized placebo-controlled trials. J Clin Psychiatry. 2011 Nov;72(11):1503-14NICE (2011). Clinical Guideline113- Generalised <strong>Anxiety</strong> Disorder in adults. Available at:http://guidance.nice.org.uk/CG113/Guidance/pdf/English [Accessed 1/4/2014]Rickels, K. et al. 2012. Adjunctive therapy with pregabaline in generalised anxiety disorder patients with partialresponse to SSRI or SNRI treatment. Int Clin Psychopharmacology. 27:142-150NICE (2013) Evidence summary- unlicensed or off-label medicine (ESUOM12): Generalised anxiety disorder.Available from: http://publications.nice.org.uk/esuom12-generalised-anxiety-disorder-quetiapine-esuom12[Accessed 30/4/14]YYN<strong>PG11</strong> – Pharmacological Treatment of <strong>Anxiety</strong> <strong>Spectrum</strong> & <strong>Related</strong> <strong>Disorders</strong>Approved by Drug <strong>and</strong> Therapeutics Committee: June 2014Review date: June 2016 Page 6 of 13