CHE 230 Exam 1 _Fall 2010 - Department of Chemistry - Illinois ...
CHE 230 Exam 1 _Fall 2010 - Department of Chemistry - Illinois ...
CHE 230 Exam 1 _Fall 2010 - Department of Chemistry - Illinois ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
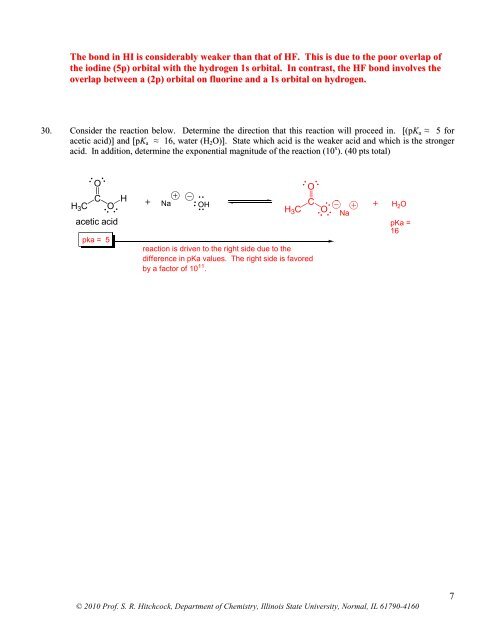
The bond in HI is considerably weaker than that <strong>of</strong> HF. This is due to the poor overlap <strong>of</strong>the iodine (5p) orbital with the hydrogen 1s orbital. In contrast, the HF bond involves theoverlap between a (2p) orbital on fluorine and a 1s orbital on hydrogen.30. Consider the reaction below. Determine the direction that this reaction will proceed in. [(pK a ≈ 5 foracetic acid)] and [pK a ≈ 16, water (H 2 O)]. State which acid is the weaker acid and which is the strongeracid. In addition, determine the exponential magnitude <strong>of</strong> the reaction (10 x ). (40 pts total)OH 3 CCOHacetic acidpka = 5+NaOHH 3 Creaction is driven to the right side due to thedifference in pKa values. The right side is favoredby a factor <strong>of</strong> 10 11 .OCONa+ H 2 OpKa =16© <strong>2010</strong> Pr<strong>of</strong>. S. R. Hitchcock, <strong>Department</strong> <strong>of</strong> <strong>Chemistry</strong>, <strong>Illinois</strong> State University, Normal, IL 61790-41607