Full Text (PDF) - Soil Science Society of America
Full Text (PDF) - Soil Science Society of America
Full Text (PDF) - Soil Science Society of America
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
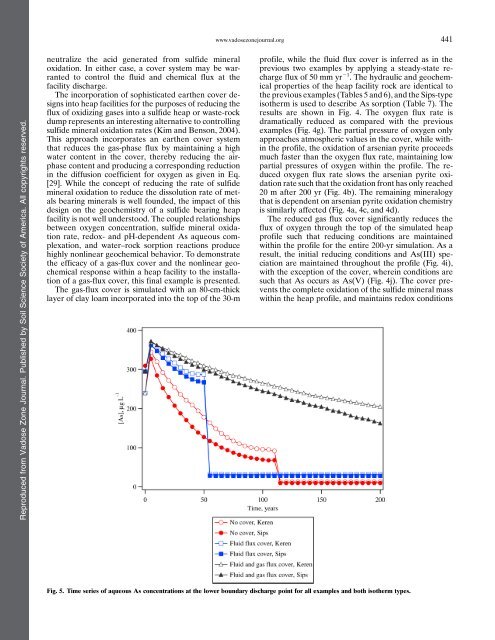
www.vadosezonejournal.org441Reproduced from Vadose Zone Journal. Published by <strong>Soil</strong> <strong>Science</strong> <strong>Society</strong> <strong>of</strong> <strong>America</strong>. All copyrights reserved.neutralize the acid generated from sulfide mineraloxidation. In either case, a cover system may be warrantedto control the fluid and chemical flux at thefacility discharge.The incorporation <strong>of</strong> sophisticated earthen cover designsinto heap facilities for the purposes <strong>of</strong> reducing theflux <strong>of</strong> oxidizing gases into a sulfide heap or waste-rockdump represents an interesting alternative to controllingsulfide mineral oxidation rates (Kim and Benson, 2004).This approach incorporates an earthen cover systemthat reduces the gas-phase flux by maintaining a highwater content in the cover, thereby reducing the airphasecontent and producing a corresponding reductionin the diffusion coefficient for oxygen as given in Eq.[29]. While the concept <strong>of</strong> reducing the rate <strong>of</strong> sulfidemineral oxidation to reduce the dissolution rate <strong>of</strong> metalsbearing minerals is well founded, the impact <strong>of</strong> thisdesign on the geochemistry <strong>of</strong> a sulfide bearing heapfacility is not well understood. The coupled relationshipsbetween oxygen concentration, sulfide mineral oxidationrate, redox- and pH-dependent As aqueous complexation,and water–rock sorption reactions producehighly nonlinear geochemical behavior. To demonstratethe efficacy <strong>of</strong> a gas-flux cover and the nonlinear geochemicalresponse within a heap facility to the installation<strong>of</strong> a gas-flux cover, this final example is presented.The gas-flux cover is simulated with an 80-cm-thicklayer <strong>of</strong> clay loam incorporated into the top <strong>of</strong> the 30-mpr<strong>of</strong>ile, while the fluid flux cover is inferred as in theprevious two examples by applying a steady-state rechargeflux <strong>of</strong> 50 mm yr 21 . The hydraulic and geochemicalproperties <strong>of</strong> the heap facility rock are identical tothe previous examples (Tables 5 and 6), and the Sips-typeisotherm is used to describe As sorption (Table 7). Theresults are shown in Fig. 4. The oxygen flux rate isdramatically reduced as compared with the previousexamples (Fig. 4g). The partial pressure <strong>of</strong> oxygen onlyapproaches atmospheric values in the cover, while withinthe pr<strong>of</strong>ile, the oxidation <strong>of</strong> arsenian pyrite proceedsmuch faster than the oxygen flux rate, maintaining lowpartial pressures <strong>of</strong> oxygen within the pr<strong>of</strong>ile. The reducedoxygen flux rate slows the arsenian pyrite oxidationrate such that the oxidation front has only reached20 m after 200 yr (Fig. 4b). The remaining mineralogythat is dependent on arsenian pyrite oxidation chemistryis similarly affected (Fig. 4a, 4c, and 4d).The reduced gas flux cover significantly reduces theflux <strong>of</strong> oxygen through the top <strong>of</strong> the simulated heappr<strong>of</strong>ile such that reducing conditions are maintainedwithin the pr<strong>of</strong>ile for the entire 200-yr simulation. As aresult, the initial reducing conditions and As(III) speciationare maintained throughout the pr<strong>of</strong>ile (Fig. 4i),with the exception <strong>of</strong> the cover, wherein conditions aresuch that As occurs as As(V) (Fig. 4j). The cover preventsthe complete oxidation <strong>of</strong> the sulfide mineral masswithin the heap pr<strong>of</strong>ile, and maintains redox conditionsFig. 5. Time series <strong>of</strong> aqueous As concentrations at the lower boundary discharge point for all examples and both isotherm types.