NSC94-EPA-Z-008-004
NSC94-EPA-Z-008-004
NSC94-EPA-Z-008-004
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
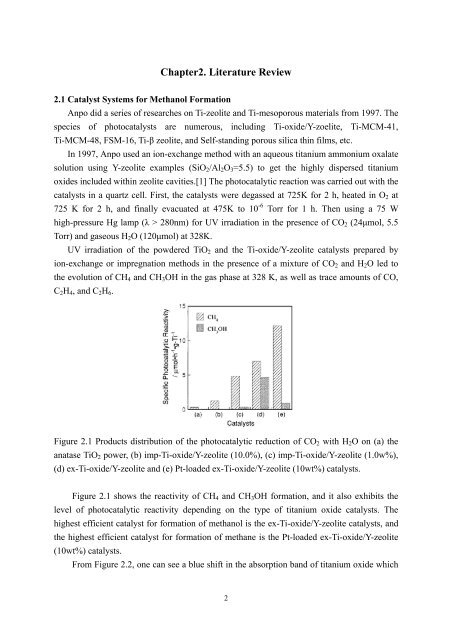
Chapter2. Literature Review2.1 Catalyst Systems for Methanol FormationAnpo did a series of researches on Ti-zeolite and Ti-mesoporous materials from 1997. Thespecies of photocatalysts are numerous, including Ti-oxide/Y-zoelite, Ti-MCM-41,Ti-MCM-48, FSM-16, Ti-β zeolite, and Self-standing porous silica thin films, etc.In 1997, Anpo used an ion-exchange method with an aqueous titanium ammonium oxalatesolution using Y-zeolite examples (SiO 2 /Al 2 O 3 =5.5) to get the highly dispersed titaniumoxides included within zeolite cavities.[1] The photocatalytic reaction was carried out with thecatalysts in a quartz cell. First, the catalysts were degassed at 725K for 2 h, heated in O 2 at725 K for 2 h, and finally evacuated at 475K to 10 -6 Torr for 1 h. Then using a 75 Whigh-pressure Hg lamp (λ > 280nm) for UV irradiation in the presence of CO 2 (24μmol, 5.5Torr) and gaseous H 2 O (120μmol) at 328K.UV irradiation of the powdered TiO 2 and the Ti-oxide/Y-zeolite catalysts prepared byion-exchange or impregnation methods in the presence of a mixture of CO 2 and H 2 O led tothe evolution of CH 4 and CH 3 OH in the gas phase at 328 K, as well as trace amounts of CO,C 2 H 4 , and C 2 H 6 .Figure 2.1 Products distribution of the photocatalytic reduction of CO 2 with H 2 O on (a) theanatase TiO 2 power, (b) imp-Ti-oxide/Y-zeolite (10.0%), (c) imp-Ti-oxide/Y-zeolite (1.0w%),(d) ex-Ti-oxide/Y-zeolite and (e) Pt-loaded ex-Ti-oxide/Y-zeolite (10wt%) catalysts.Figure 2.1 shows the reactivity of CH 4 and CH 3 OH formation, and it also exhibits thelevel of photocatalytic reactivity depending on the type of titanium oxide catalysts. Thehighest efficient catalyst for formation of methanol is the ex-Ti-oxide/Y-zeolite catalysts, andthe highest efficient catalyst for formation of methane is the Pt-loaded ex-Ti-oxide/Y-zeolite(10wt%) catalysts.From Figure 2.2, one can see a blue shift in the absorption band of titanium oxide which2