Efficacy and Safety of Ursodeoxycholic Acid Versus Cholestyramine ...
Efficacy and Safety of Ursodeoxycholic Acid Versus Cholestyramine ...
Efficacy and Safety of Ursodeoxycholic Acid Versus Cholestyramine ...
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
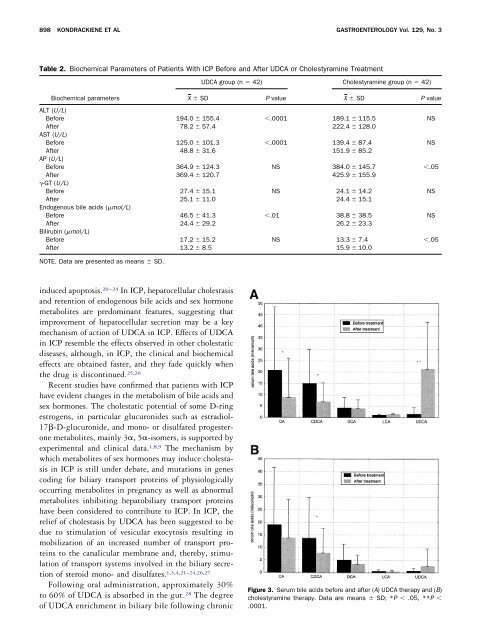
898 KONDRACKIENE ET AL GASTROENTEROLOGY Vol. 129, No. 3Table 2. Biochemical Parameters <strong>of</strong> Patients With ICP Before <strong>and</strong> After UDCA or <strong>Cholestyramine</strong> TreatmentUDCA group (n 42) <strong>Cholestyramine</strong> group (n 42)Biochemical parametersX SD P value X SD P valueALT (U/L)Before 194.0 155.4 .0001 189.1 115.5 NSAfter 78.2 57.4 222.4 128.0AST (U/L)Before 125.0 101.3 .0001 139.4 87.4 NSAfter 48.8 31.6 151.9 85.2AP (U/L)Before 364.9 124.3 NS 384.0 145.7 .05After 369.4 120.7 425.9 155.9-GT (U/L)Before 27.4 15.1 NS 24.1 14.2 NSAfter 25.1 11.0 24.4 15.1Endogenous bile acids (mol/L)Before 46.5 41.3 .01 38.8 38.5 NSAfter 24.4 29.2 26.2 23.3Bilirubin (mol/L)Before 17.2 15.2 NS 13.3 7.4 .05After 13.2 8.5 15.9 10.0NOTE. Data are presented as means SD.induced apoptosis. 20–24 In ICP, hepatocellular cholestasis<strong>and</strong> retention <strong>of</strong> endogenous bile acids <strong>and</strong> sex hormonemetabolites are predominant features, suggesting thatimprovement <strong>of</strong> hepatocellular secretion may be a keymechanism <strong>of</strong> action <strong>of</strong> UDCA in ICP. Effects <strong>of</strong> UDCAin ICP resemble the effects observed in other cholestaticdiseases, although, in ICP, the clinical <strong>and</strong> biochemicaleffects are obtained faster, <strong>and</strong> they fade quickly whenthe drug is discontinued. 25,26Recent studies have confirmed that patients with ICPhave evident changes in the metabolism <strong>of</strong> bile acids <strong>and</strong>sex hormones. The cholestatic potential <strong>of</strong> some D-ringestrogens, in particular glucuronides such as estradiol-17-D-glucuronide, <strong>and</strong> mono- or disulfated progesteronemetabolites, mainly 3, 5-isomers, is supported byexperimental <strong>and</strong> clinical data. 1,8,9 The mechanism bywhich metabolites <strong>of</strong> sex hormones may induce cholestasisin ICP is still under debate, <strong>and</strong> mutations in genescoding for biliary transport proteins <strong>of</strong> physiologicallyoccurring metabolites in pregnancy as well as abnormalmetabolites inhibiting hepatobiliary transport proteinshave been considered to contribute to ICP. In ICP, therelief <strong>of</strong> cholestasis by UDCA has been suggested to bedue to stimulation <strong>of</strong> vesicular exocytosis resulting inmobilization <strong>of</strong> an increased number <strong>of</strong> transport proteinsto the canalicular membrane <strong>and</strong>, thereby, stimulation<strong>of</strong> transport systems involved in the biliary secretion<strong>of</strong> steroid mono- <strong>and</strong> disulfates. 1,3,4,21–24,26,27Following oral administration, approximately 30%to 60% <strong>of</strong> UDCA is absorbed in the gut. 28 The degree<strong>of</strong> UDCA enrichment in biliary bile following chronicFigure 3. Serum bile acids before <strong>and</strong> after (A) UDCA therapy <strong>and</strong> (B)cholestyramine therapy. Data are means SD; *P .05, **P .0001.