- Page 1 and 2: UNIVERSITY OF KWAZULU-NATALTHE PHYT
- Page 3 and 4: P a g e | iiiABSTRACTThis work is a
- Page 5 and 6: P a g e | vSUMMARY OF COMPOUNDS ISO
- Page 7 and 8: P a g e | vii5'3'4'4' 5'3'2'O1'OCH
- Page 9 and 10: P a g e | ixOOOOOOCOCH 312345678910
- Page 11 and 12: P a g e | xidddHzMestIRMptlcdoublet
- Page 13 and 14: P a g e | xiiiDECLARATION 2-PUBLICA
- Page 15: P a g e | xvACKNOWLEDGEMENTSI would
- Page 18 and 19: P a g e | xviii1.2.4. A phytochemic
- Page 20 and 21: P a g e | xxExperimental Section ..
- Page 22 and 23: P a g e | 2stems are upright and st
- Page 24 and 25: P a g e | 4diseases, liver protecti
- Page 26 and 27: P a g e | 6anti-amoebic or antihelm
- Page 28 and 29: P a g e | 8V. karaguensis antibacte
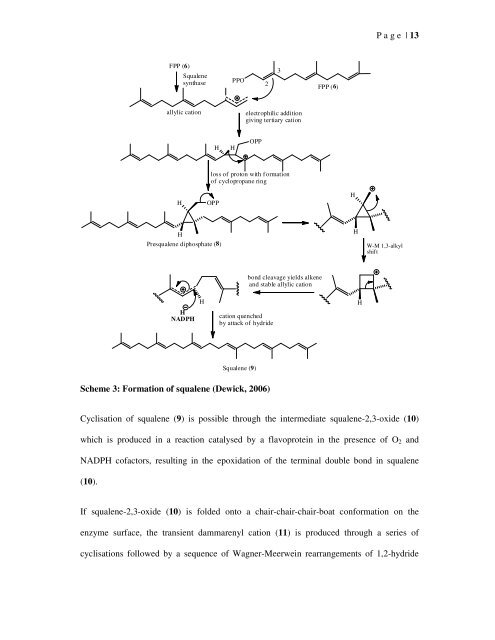
- Page 30 and 31: P a g e | 10In plants, the enzymes
- Page 34 and 35: P a g e | 14and 1,2-methyl migratio
- Page 36 and 37: P a g e | 16HH1,2-alkyl shiftfive m
- Page 38 and 39: P a g e | 18In addition, α-amyrin
- Page 40 and 41: P a g e | 203030AcO22313 4525119102
- Page 42 and 43: P a g e | 22Table 3: Tritepenoids c
- Page 44 and 45: P a g e | 24plaque and also by disl
- Page 46 and 47: P a g e | 26Table 5 Biological acti
- Page 48 and 49: P a g e | 28The quinoline ring is f
- Page 50 and 51: P a g e | 30COSCoAMalonyl-CoAclaise
- Page 52 and 53: P a g e | 32OCH 3OCH 3OCH 3O654a43a
- Page 54 and 55: P a g e | 34R 35'O65 471R 28NR 1R63
- Page 56 and 57: P a g e | 36O R 1R 2788a91a12R 6106
- Page 58 and 59: P a g e | 38232220 OO182112 171 OO2
- Page 60 and 61: P a g e | 40HH21HOH2324OHO25C-21, C
- Page 62 and 63: P a g e | 42OOOOOOAcnomilinOOOOOOOO
- Page 64 and 65: P a g e | 44ROOCOOHOHOH 3 COCH 3H 3
- Page 66 and 67: P a g e | 46H 3 COOH 3 COOONN111 11
- Page 68 and 69: P a g e | 48ReferencesAbosi, A. O.,
- Page 70 and 71: P a g e | 50Bardón, A., Catalán,
- Page 72 and 73: P a g e | 52Chhabra, S. C., Mahunna
- Page 74 and 75: P a g e | 54Funk, V. A., Bayer, R.
- Page 76 and 77: P a g e | 56Haensel, R., Cybulski,
- Page 78 and 79: P a g e | 58Kupchan, S. M., Hemingw
- Page 80 and 81: P a g e | 60Moundipa, P. F., Flore,
- Page 82 and 83:
P a g e | 62Parekh, J., Chanda, S.
- Page 84 and 85:
P a g e | 64Sy, G. Y., Nongonierma,
- Page 86 and 87:
P a g e | 66CHAPTER TWOTRITERPENOID
- Page 88 and 89:
P a g e | 68the genus Vernonia are
- Page 90 and 91:
P a g e | 70dichloromethane in hexa
- Page 92 and 93:
P a g e | 72the test compounds that
- Page 94 and 95:
P a g e | 74Compound 9 was isolated
- Page 96 and 97:
P a g e | 76saprophyticus, and 0.5m
- Page 98 and 99:
P a g e | 783.02.5UNTREATEDSUB-MICM
- Page 100 and 101:
P a g e | 80ACKNOWLEDGEMENTSThe aut
- Page 102 and 103:
P a g e | 82Igoli OJ, Gray IA (2008
- Page 104 and 105:
P a g e | 84Morales-Escobar L, Brac
- Page 106 and 107:
P a g e | 86intestinal colics and c
- Page 108 and 109:
P a g e | 88Determination of minimu
- Page 110 and 111:
P a g e | 90overlapped in the olefi
- Page 112 and 113:
P a g e | 92Table 1. 1 H and 13 C N
- Page 114 and 115:
P a g e | 94structure with the cyto
- Page 116 and 117:
P a g e | 96Kull DR, Pfander H, (19
- Page 118 and 119:
P a g e | 98furoquinoline as well a
- Page 120 and 121:
P a g e | 100The characteristic ABX
- Page 122 and 123:
P a g e | 10215.625 µg mL -1 , nko
- Page 124 and 125:
P a g e | 104with known antioxidant
- Page 126 and 127:
P a g e | 106(3H, s, OCH 3 ), 3.50
- Page 128 and 129:
P a g e | 1088. Karou D, Savadogo A
- Page 130 and 131:
P a g e | 11023. Shirwakar A, Shirw
- Page 132 and 133:
P a g e | 112IntroductionThere are
- Page 134 and 135:
P a g e | 114HOHOH 3 CO764385OH910O
- Page 136 and 137:
P a g e | 116This is the first repo
- Page 138 and 139:
P a g e | 11813 C NMR spectra). The
- Page 140 and 141:
P a g e | 1203.18 (1H, d, J = 9.15
- Page 142 and 143:
P a g e | 122AcknowledgmentsWe than
- Page 144 and 145:
P a g e | 124Didry, N., Seidel, V.,
- Page 146 and 147:
P a g e | 126Poitou, F., Masotti, V
- Page 148 and 149:
P a g e | 128method. The current co
- Page 150 and 151:
P a g e | 130subjected to antioxida
- Page 152 and 153:
P a g e | 1323H-29 with C-4 and C-5
- Page 154 and 155:
P a g e | 134O229119103428H3COCO21H
- Page 156 and 157:
P a g e | 136to the carbonyl group,
- Page 158 and 159:
P a g e | 138methylaromadenrin (9)
- Page 160 and 161:
P a g e | 140them 29 . The flavanol
- Page 162 and 163:
P a g e | 142and sesquiterpene may
- Page 164 and 165:
P a g e | 144further using dichloro
- Page 166 and 167:
P a g e | 146ANTIOXIDANT ACTIVITYDe
- Page 168 and 169:
P a g e | 148Chicago, IL, USA). The
- Page 170 and 171:
P a g e | 15013. Cheplogoi, P. K.;
- Page 172 and 173:
P a g e | 152CHAPTER SEVENSUMMARY A
- Page 174 and 175:
P a g e | 154also be used as an ant
- Page 176 and 177:
P a g e | 156The isolation of limon
- Page 178:
P a g e | 158SUPPORTING INFORMATION














![SYNTHESIS AND ANTI-HIV ACTIVITY OF [d4U]-SPACER-[HI-236 ...](https://img.yumpu.com/30883288/1/190x245/synthesis-and-anti-hiv-activity-of-d4u-spacer-hi-236-.jpg?quality=85)
