Meeting No-10 dated 08.06.2005.pdf - Directorate General of ...
Meeting No-10 dated 08.06.2005.pdf - Directorate General of ...
Meeting No-10 dated 08.06.2005.pdf - Directorate General of ...
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
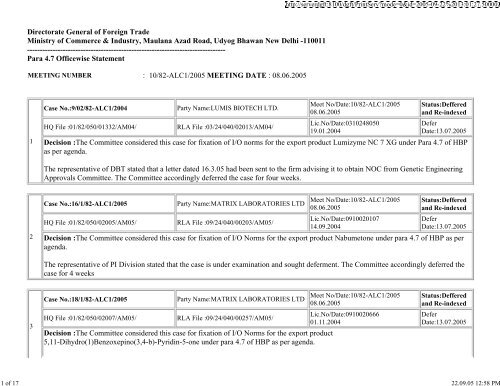
http://serverdgft:8<strong>10</strong>0/dgft/PrintServ?mode=0&id=2005-09-22%2013:01:27.5000021 <strong>of</strong> 17 22.09.05 12:58 PM<strong>Directorate</strong> <strong>General</strong> <strong>of</strong> Foreign TradeMinistry <strong>of</strong> Commerce & Industry, Maulana Azad Road, Udyog Bhawan New Delhi -1<strong>10</strong>011------------------------------------------------------------------------------Para 4.7 Officewise StatementMEETING NUMBER : <strong>10</strong>/82-ALC1/2005 MEETING DATE : 08.06.20051Case <strong>No</strong>.:9/02/82-ALC1/2004HQ File :01/82/050/01332/AM04/Party Name:LUMIS BIOTECH LTD.RLA File :03/24/040/02013/AM04/Meet <strong>No</strong>/Date:<strong>10</strong>/82-ALC1/200508.06.2005Lic.<strong>No</strong>/Date:03<strong>10</strong>24805019.01.2004Status:Defferedand Re-indexedDeferDate:13.07.2005Decision :The Committee considered this case for fixation <strong>of</strong> I/O norms for the export product Lumizyme NC 7 XG under Para 4.7 <strong>of</strong> HBPas per agenda.The representative <strong>of</strong> DBT stated that a letter <strong>dated</strong> 16.3.05 had been sent to the firm advising it to obtain NOC from Genetic EngineeringApprovals Committee. The Committee accordingly deferred the case for four weeks.2Case <strong>No</strong>.:16/1/82-ALC1/2005 Party Name:MATRIX LABORATORIES LTDHQ File :01/82/050/02005/AM05/RLA File :09/24/040/00203/AM05/Meet <strong>No</strong>/Date:<strong>10</strong>/82-ALC1/200508.06.2005Lic.<strong>No</strong>/Date:09<strong>10</strong>020<strong>10</strong>714.09.2004Status:Defferedand Re-indexedDeferDate:13.07.2005Decision :The Committee considered this case for fixation <strong>of</strong> I/O <strong>No</strong>rms for the export product Nabumetone under para 4.7 <strong>of</strong> HBP as peragenda.The representative <strong>of</strong> PI Division stated that the case is under examination and sought deferment. The Committee accordingly deferred thecase for 4 weeks3Case <strong>No</strong>.:18/1/82-ALC1/2005 Party Name:MATRIX LABORATORIES LTDHQ File :01/82/050/02007/AM05/RLA File :09/24/040/00257/AM05/Decision :The Committee considered this case for fixation <strong>of</strong> I/O <strong>No</strong>rms for the export product5,11-Dihydro(1)Benzoxepino(3,4-b)-Pyridin-5-one under para 4.7 <strong>of</strong> HBP as per agenda.Meet <strong>No</strong>/Date:<strong>10</strong>/82-ALC1/200508.06.2005Lic.<strong>No</strong>/Date:09<strong>10</strong>02066601.11.2004Status:Defferedand Re-indexedDeferDate:13.07.2005
http://serverdgft:8<strong>10</strong>0/dgft/PrintServ?mode=0&id=2005-09-22%2013:01:27.5000022 <strong>of</strong> 17 22.09.05 12:58 PMThe representative <strong>of</strong> PI Division stated that the case is under examination and sought deferment. The Committee accordingly deferred thecase for 4 weeks.4Case <strong>No</strong>.:25/37/82-ALC1/2004HQ File :01/82/050/01415/AM05/Party Name:KDL BIOTECH LIMITEDRLA File :03/24/040/01364/AM05/Meet <strong>No</strong>/Date:<strong>10</strong>/82-ALC1/200508.06.2005Lic.<strong>No</strong>/Date:03<strong>10</strong>30198811.11.2004Status:Defferedand Re-indexedDeferDate:13.07.2005Decision :The Committee considered this case for fixation <strong>of</strong> I/O norms for the export product Immobilised Pencilling-G- Amidase Enzymeunder para 4.7 <strong>of</strong> HBP as per agenda.The Committee had referred this case to DBT in the Advance Licensing Committee meeting held on 4.5.05. The representative <strong>of</strong> DBTstated in the meeting that the case pertains to PI Division. The committee accordingly decided to refer the case to PI Division and deferredthe case for 4 weeks.Case <strong>No</strong>.:33/40/82-ALC1/2004HQ File :01/82/050/01357/AM05/Party Name:FLEMING LABORATORIESLTD.,RLA File :09/24/040/00265/AM05/Meet <strong>No</strong>/Date:<strong>10</strong>/82-ALC1/200508.06.2005Lic.<strong>No</strong>/Date:09<strong>10</strong>02079816.11.2004Status:CaseApprovedDefer Date:Decision :Decision :The Committee considered this case for fixation <strong>of</strong> I/O norms for the export product Ketotifen Hydrogen FumarateEP/Ketotifen Fumarate under para 4.7 <strong>of</strong> HBP as per agenda.5The Committee in light <strong>of</strong> the written comments <strong>of</strong> the D/o C&PC forwarded vide their U.O.<strong>No</strong>. 35011/16/2005/PI-III <strong>dated</strong> 12.5.05decided to fix I/O norms for the purpose <strong>of</strong> Para 4.7 <strong>of</strong> HBP only as under:Export productKetotifen Hydrogen Fumarate EP/Ketotifen Fumarate ---1kgImport items1. <strong>10</strong>-Methoxy-4H-Benzo(4,5)Cyclohepta(1,2-b)thiophen-4-one-------1.179 kgRLA concerned may take further necessary action under para 4.7 <strong>of</strong> HBP as per above decision <strong>of</strong> ALC.6 Case <strong>No</strong>.:2/33/82-ALC1/2004 Party Name:MICRO INKS LTD.Meet <strong>No</strong>/Date:<strong>10</strong>/82-ALC1/200508.06.2005Status:Defferedand Re-indexed
http://serverdgft:8<strong>10</strong>0/dgft/PrintServ?mode=0&id=2005-09-22%2013:01:27.5000023 <strong>of</strong> 17 22.09.05 12:58 PMHQ File :01/82/050/01455/AM05/RLA File :03/24/040/01848/AM05/Lic.<strong>No</strong>/Date:03<strong>10</strong>30293819.11.2004DeferDate:<strong>10</strong>.08.2005Decision :The Committee considered this case for fixation <strong>of</strong> I/O <strong>No</strong>rms for the export product Offset Printing Ink Black under Para 4.7 <strong>of</strong>HBP as per agenda.The Committee noted that a team is likely to visit the manufacturing unit to evaluate the requirement <strong>of</strong> input output norms after analysis <strong>of</strong>information called for from the firm vide letter <strong>dated</strong> 16.5.05. The Committee accordingly, decided to defer this case for 8 weeksCase <strong>No</strong>.:34/40/82-ALC1/2004HQ File :01/82/050/01449/AM05/Party Name:M/S.ENAL DRUGS PVT LTD.,RLA File :09/24/040/00267/AM05/Meet <strong>No</strong>/Date:<strong>10</strong>/82-ALC1/200508.06.2005Lic.<strong>No</strong>/Date:09<strong>10</strong>02084819.11.2004Status:CaseApprovedDefer Date:Decision :Decision : The Committee considered this case for fixation <strong>of</strong> I/O norms for the export product under para 4.7 <strong>of</strong> HBP as peragenda.7The Committee in light <strong>of</strong> the written comments <strong>of</strong> the Deptt. <strong>of</strong> C&PC forwarded vide their U.O.<strong>No</strong>. 35011/17/2005 PI-III <strong>dated</strong> 12.5.05decided to fix I/O <strong>No</strong>rms for the export product for the purpose <strong>of</strong> para 4.7 <strong>of</strong> HBP only as under:Export product2,3 Dimethyl-4-Nitro Pyridine N-Oxide--------1kgImport items1. 2,3 Lutidine----------------0.85 kg2. Methylene Chloride (MDC)----1.175 kg3. Iso Propyl Alcohol-----0.46 kgRLA concerned may take further necessary action under para 4.7 <strong>of</strong> HBP as per above decision <strong>of</strong> ALC.8Case <strong>No</strong>.:4/33/82-ALC1/2004HQ File :01/82/050/01458/AM05/Party Name:MICRO INKS LTD.RLA File :03/24/040/01850/AM05/Meet <strong>No</strong>/Date:<strong>10</strong>/82-ALC1/200508.06.2005Lic.<strong>No</strong>/Date:03<strong>10</strong>30311622.11.2004Status:Defferedand Re-indexedDeferDate:<strong>10</strong>.08.2005Decision :The Committee considered this case for fixation <strong>of</strong> I/O <strong>No</strong>rms for the export product Oil Soluble Phenolic Resin Based Varnishunder Para 4.7 <strong>of</strong> HBP as per agenda.The Committee noted that a team is likely to visit the manufacturing unit to evaluate the requirement <strong>of</strong> input output norms after analysis <strong>of</strong>
http://serverdgft:8<strong>10</strong>0/dgft/PrintServ?mode=0&id=2005-09-22%2013:01:27.5000024 <strong>of</strong> 17 22.09.05 12:58 PMinformation called for from the firm vide letter <strong>dated</strong> 16.5.05. The Committee accordingly, decided to defer this case for 8 weeks.9Case <strong>No</strong>.:7/33/82-ALC1/2004HQ File :01/82/050/01470/AM05/Party Name:MICRO INKS LTD.RLA File :03/24/040/01849/AM05/Meet <strong>No</strong>/Date:<strong>10</strong>/82-ALC1/200508.06.2005Lic.<strong>No</strong>/Date:03<strong>10</strong>30332123.11.2004Status:Defferedand Re-indexedDeferDate:<strong>10</strong>.08.2005Decision :The Committee considered this case for fixation <strong>of</strong> I/O <strong>No</strong>rms for the export product Oil Soluble Phenolic Resin Based Varnishunder Para 4.7 <strong>of</strong> HBP as per agenda.The Committee noted that a team is likely to visit the manufacturing unit to evaluate the requirement <strong>of</strong> input output norms after analysis <strong>of</strong>information called for from the firm vide letter <strong>dated</strong> 16.5.05. The Committee accordingly, decided to defer this case for 8 weeks.<strong>10</strong>Case <strong>No</strong>.:8/33/82-ALC1/2004HQ File :01/82/050/01472/AM05/Party Name:MICRO INKS LTD.RLA File :03/24/040/01875/AM05/Meet <strong>No</strong>/Date:<strong>10</strong>/82-ALC1/200508.06.2005Lic.<strong>No</strong>/Date:03<strong>10</strong>30337723.11.2004Status:Defferedand Re-indexedDeferDate:<strong>10</strong>.08.2005Decision :The Committee considered this case for fixation <strong>of</strong> I/O <strong>No</strong>rms for the export product Oil Soluble Phenolic Resin Based Varnishunder Para 4.7 <strong>of</strong> HBP as per agenda.The Committee noted that a team is likely to visit the manufacturing unit to evaluate the requirement <strong>of</strong> input output norms after analysis <strong>of</strong>information called for from the firm vide letter <strong>dated</strong> 16.5.05. The Committee accordingly, decided to defer this case for 8 weeks11Case <strong>No</strong>.:11/33/82-ALC1/2004HQ File :01/82/050/01484/AM05/Party Name:MICRO INKS LTD.RLA File :03/24/040/01874/AM05/Meet <strong>No</strong>/Date:<strong>10</strong>/82-ALC1/200508.06.2005Lic.<strong>No</strong>/Date:03<strong>10</strong>30368124.11.2004Status:Defferedand Re-indexedDeferDate:<strong>10</strong>.08.2005Decision :The Committee considered this case for fixation <strong>of</strong> I/O <strong>No</strong>rms for the export product Oil Soluble Phenolic Resin Based Varnishunder Para 4.7 <strong>of</strong> HBP as per agenda.The Committee noted that a team is likely to visit the manufacturing unit to evaluate the requirement <strong>of</strong> input output norms after analysis <strong>of</strong>information called for from the firm vide letter <strong>dated</strong> 16.5.05. The Committee accordingly, decided to defer this case for 8 weeks12Case <strong>No</strong>.:12/33/82-ALC1/2004Party Name:MICRO INKS LTD.Meet <strong>No</strong>/Date:<strong>10</strong>/82-ALC1/200508.06.2005Status:Defferedand Re-indexed
http://serverdgft:8<strong>10</strong>0/dgft/PrintServ?mode=0&id=2005-09-22%2013:01:27.5000025 <strong>of</strong> 17 22.09.05 12:58 PMHQ File :01/82/050/01485/AM05/RLA File :03/24/040/01888/AM05/Lic.<strong>No</strong>/Date:03<strong>10</strong>30368224.11.2004DeferDate:<strong>10</strong>.08.2005Decision :The Committee considered this case for fixation <strong>of</strong> I/O <strong>No</strong>rms for the export product Oil Soluble Phenolic Resin Based Varnishunder Para 4.7 <strong>of</strong> HBP as per agenda.The Committee noted that a team is likely to visit the manufacturing unit to evaluate the requirement <strong>of</strong> input output norms after analysis <strong>of</strong>information called for from the firm vide letter <strong>dated</strong> 16.5.05. The Committee accordingly, decided to defer this case for 8 weeksCase <strong>No</strong>.:2/1/82-ALC1/2005HQ File :01/82/050/01496/AM05/Party Name:DIVIS LABORATORIES LTD.,RLA File :09/24/040/00281/AM05/Meet <strong>No</strong>/Date:<strong>10</strong>/82-ALC1/200508.06.2005Lic.<strong>No</strong>/Date:09<strong>10</strong>02099302.12.2004Status:CaseApprovedDefer Date:Decision :The Committee considered this case for fixation <strong>of</strong> I/O norms for the export product Tamsulosin Hcl under para 4.7 <strong>of</strong> HBP as peragenda.The Committee in light <strong>of</strong> the written comments <strong>of</strong> the Deptt. <strong>of</strong> C&PC forwarded vide their U.O.<strong>No</strong>. 35011/183/2004-PI-III <strong>dated</strong> 26.5.05decided to fix I/O <strong>No</strong>rms for the export product for the purpose <strong>of</strong> para 4.7 <strong>of</strong> HBP only as under:13Export ProductTamsulosin Hcl--------1kgImport items1. 4-Methoxy Phenyl Acetone----8.81 kg2. Toluene-------16.50 kg3. Tetra Hydro Furan-----18.71 kg4. Di Isopropyl Ether-----5.61 kg5. Methanol-----45.97 kg6. Chloro Acetonitrile---------2.60 kg7. Acetonitrile-------4.40 kgRLA concerned may take further necessary action under para 4.7 <strong>of</strong> HBP as per above decision <strong>of</strong> ALC.
http://serverdgft:8<strong>10</strong>0/dgft/PrintServ?mode=0&id=2005-09-22%2013:01:27.5000026 <strong>of</strong> 17 22.09.05 12:58 PM14Case <strong>No</strong>.:3/1/82-ALC1/2005HQ File :01/82/050/01546/AM05/Party Name:DR REDDYS LABORATORIESLTDRLA File :09/24/040/00290/AM05/Meet <strong>No</strong>/Date:<strong>10</strong>/82-ALC1/200508.06.2005Lic.<strong>No</strong>/Date:09<strong>10</strong>02<strong>10</strong>7009.12.2004Status:Defferedand Re-indexedDeferDate:13.07.2005Decision :The committee considered this case for fixation <strong>of</strong> I/O <strong>No</strong>rms for the export product 3-(4-Amino-3-Methylbenzyl)-7-(2-Furyl)3H-(1,2,3)-Triazolo(4,5-d)-Pyrimidine-5-Amine under Para 4.7 <strong>of</strong> HBP as per agenda.The representative <strong>of</strong> PI Division stated that the case is under examination and sought deferment. The Committee accordingly deferred thecase for 4 weeks15Case <strong>No</strong>.:8/36/82-ALC1/2004HQ File :01/82/050/01547/AM05/Party Name:EMAMI LIMITED,RLA File :02/24/040/00093/AM05/Meet <strong>No</strong>/Date:<strong>10</strong>/82-ALC1/200508.06.2005Lic.<strong>No</strong>/Date:02<strong>10</strong>072638<strong>10</strong>.12.2004Status:CaseRejectedDefer Date:Decision :Decision : The committee considered this case for fixation <strong>of</strong> I/O <strong>No</strong>rms for the export product Boroplus Antiseptic Cream underPara 4.7 <strong>of</strong> HBP as per agenda.The Committee noted that information had been called for from the firm vide letter dt. 28.3.05 and that reply had not been received. In theabsence <strong>of</strong> the information called for, it was not possible for the Committee to work out the Input Output <strong>No</strong>rms. The Committeeaccordingly rejected the case.RLA concerned may take further necessary action including cancellation <strong>of</strong> licence under Para 4.7 <strong>of</strong> HBP as per above decision <strong>of</strong> theCommittee.16Case <strong>No</strong>.:13/44/82-ALC1/2004HQ File :01/82/050/01647/AM05/Party Name:COVALENT LABORATORIESPRIVATE LIMITEDRLA File :09/24/040/00302/AM05/Meet <strong>No</strong>/Date:<strong>10</strong>/82-ALC1/200508.06.2005Lic.<strong>No</strong>/Date:09<strong>10</strong>02130130.12.2004Status:CaseApprovedDefer Date:Decision :The Committee considered this case for fixation <strong>of</strong> I/O norms for the export product under para 4.7 <strong>of</strong> HBP as per agenda.The Committee in light <strong>of</strong> the written comments <strong>of</strong> the Deptt. <strong>of</strong> C&PC forwarded vide their U.O.<strong>No</strong>. 35011/256/2005-PI-III <strong>dated</strong> 13.6.05decided to fix I/O <strong>No</strong>rms for the export product for the purpose <strong>of</strong> para 4.7 <strong>of</strong> HBP only as under:Export productCefprozil USP--------1kg
http://serverdgft:8<strong>10</strong>0/dgft/PrintServ?mode=0&id=2005-09-22%2013:01:27.5000027 <strong>of</strong> 17 22.09.05 12:58 PMImport items1. 7-Phenylacetamideo-3-chloromethyl Cephalosporanic Acid---3.753 kg2. Methylene chloride-------25.36 kg3. Di Methyl formamide---5.385 kg4. P-Hydroxy Phenyl Glicine Dane Salt-------1.05 kgRLA concerned may take further necessary action under para 4.7 <strong>of</strong> HBP as per above decision <strong>of</strong> ALC.17Case <strong>No</strong>.:22/1/82-ALC1/2005HQ File :01/82/050/01650/AM05/Party Name:ITC LIMITEDRLA File :09/24/040/00305/AM05/Meet <strong>No</strong>/Date:<strong>10</strong>/82-ALC1/200508.06.2005Lic.<strong>No</strong>/Date:09<strong>10</strong>02132703.01.2005Status:Defferedand Re-indexedDeferDate:13.07.2005Decision :The committee considered this case for fixation <strong>of</strong> I/O <strong>No</strong>rms for the export product Poly Coated Carton Board IPE under Para4.7 <strong>of</strong> HBP as per agenda.The Committee noted that a letter calling for information from the firm had been issued on 20.4.05 and decided to await reply. TheCommittee accordingly, decided to defer this case for 4 weeks.18Case <strong>No</strong>.:28/40/82-ALC1/2004HQ File :01/82/050/01672/AM05/Party Name:G S P CROP SCIENCE PVT.LTD.RLA File :08/80/040/00075/AM05/Meet <strong>No</strong>/Date:<strong>10</strong>/82-ALC1/200508.06.2005Lic.<strong>No</strong>/Date:08<strong>10</strong>04488306.01.2005Status:Defferedand Re-indexedDeferDate:13.07.2005Decision :The committee considered this case for fixation <strong>of</strong> I/O <strong>No</strong>rms for the export product Imidacloprid 20%SL (Sensor 200SL) underPara 4.7 <strong>of</strong> HBP as per agenda.The representative <strong>of</strong> Chemical Division stated that the following information had been called for vide D/oC&PC letter <strong>No</strong> 15022/6/2005-Ch.II <strong>dated</strong> 28.3.05:(i) Stagewise percentage yield on Mol Weight basis alongwith justification; (ii) Authentic/printed literature in support <strong>of</strong> percentage yield ornorms applied for import; (iii) Complete material balance, percentage loss for conversion <strong>of</strong> Imidacloprid Technical along with its purity to20% SL and composition <strong>of</strong> export item; (iv) Density <strong>of</strong> export item; and (v) Production <strong>of</strong> export item for the past two years upto March2005 and corresponding consumption <strong>of</strong> each input duly certified by Chartered Accountant. He further stated that on non-receipt <strong>of</strong>information from the firm, a reminder was issued by D/o C&PC bearing <strong>No</strong> ibid <strong>dated</strong> 27.5.05 requesting the firm to submit the requisiteinformation. The Committee accordingly decided to await information from the firm and to defer the case for four weeks.19 Case <strong>No</strong>.:51/1/82-ALC1/2005 Party Name:DIVIS LABORATORIES LTD.,Meet <strong>No</strong>/Date:<strong>10</strong>/82-ALC1/200508.06.2005Status:CaseApproved
http://serverdgft:8<strong>10</strong>0/dgft/PrintServ?mode=0&id=2005-09-22%2013:01:27.5000028 <strong>of</strong> 17 22.09.05 12:58 PMHQ File :01/82/050/01697/AM05/RLA File :09/24/040/00319/AM05/Lic.<strong>No</strong>/Date:09<strong>10</strong>02148011.01.2005Defer Date:Decision :The committee considered this case for fixation <strong>of</strong> I/O <strong>No</strong>rms for the export product Iopamidol USP under Para 4.7 <strong>of</strong> HBP as peragenda.The Committee in light <strong>of</strong> the written comments <strong>of</strong> the Deptt. <strong>of</strong> C&PC forwarded vide their U.O.<strong>No</strong>. 35011/183/2004/PI-III <strong>dated</strong> 26.5.05decided to fix I/O <strong>No</strong>rms for the export product for the purpose <strong>of</strong> para 4.7 <strong>of</strong> HBP only as under:Export productIopdamidol USP---------1kgImport items1. Isophthalic Acid---1.40 kg2. Iodine----------2.16 kg3. Methanol---------2.39 kg4. IPA----11.00 kg5. Nitromethane-----------0.48 kg6. Paraformaldehyde-----0.48 kg7. S-(-)-2-Acetoxy Propionyl Chloride--------0.85kg8. Dimethyl Formamide----0.78 kgRLA concerned may take further necessary action under para 4.7 <strong>of</strong> HBP as per above decision <strong>of</strong> ALC.The Committee noted that the firm has applied for the export product as per the quantities given under SION <strong>No</strong> A-2069. The Committeenoted that there were some omissions/typographical errors while publishing the SION at A-2069 which resulted in duplication <strong>of</strong> entries <strong>of</strong>some import items. The Committee decided to correct the SION A-2069 as per the above norms for the export product after obtainingwritten comments ffrom PI Division20Case <strong>No</strong>.:7/1/82-ALC1/2005HQ File :01/82/050/01696/AM05/Party Name:DIVIS LABORATORIES LTD.,RLA File :09/24/040/00313/AM05/Meet <strong>No</strong>/Date:<strong>10</strong>/82-ALC1/200508.06.2005Lic.<strong>No</strong>/Date:09<strong>10</strong>02147611.01.2005Status:Defferedand Re-indexedDeferDate:13.07.2005Decision :The Committee considered this case for fixation <strong>of</strong> I/O norms for the export product under para 4.7 <strong>of</strong> HBP as per agenda.The representative <strong>of</strong> PI Division stated that the application <strong>of</strong> the firm sent to him earlier was not traceable. The Committee accordinglydecided to send him the application and to defer the case for four weeks.
http://serverdgft:8<strong>10</strong>0/dgft/PrintServ?mode=0&id=2005-09-22%2013:01:27.5000029 <strong>of</strong> 17 22.09.05 12:58 PM21Case <strong>No</strong>.:2/42/82-ALC1/2004HQ File :01/82/050/01715/AM05/Party Name:EMAMI LIMITED,RLA File :02/24/040/00235/AM05/Meet <strong>No</strong>/Date:<strong>10</strong>/82-ALC1/200508.06.2005Lic.<strong>No</strong>/Date:02<strong>10</strong>07401114.01.2005Status:CaseRejectedDefer Date:Decision :The committee considered this case for fixation <strong>of</strong> I/O <strong>No</strong>rms for the export product Himani Gold Turmeric Saffron Soap underPara 4.7 <strong>of</strong> HBP as per agenda.The Committee noted that information had been sought from the firm vide letter dt. 15.3.05 and that reply from the firm had not beenreceived. In the absence <strong>of</strong> the information called for from the firm, it was not possible for the Committee to work out the I/O norms. TheCommittee accordingly decided to reject this case.RLA concerned may take further necessary action including cancellation <strong>of</strong> licence under Para 4.7 <strong>of</strong> HBP as per above decision <strong>of</strong> theCommittee.22Case <strong>No</strong>.:9/1/82-ALC1/2005HQ File :01/82/050/01726/AM05/Party Name:DIVIS LABORATORIES LTD.,RLA File :09/24/040/00323/AM05/Meet <strong>No</strong>/Date:<strong>10</strong>/82-ALC1/200508.06.2005Lic.<strong>No</strong>/Date:09<strong>10</strong>02156518.01.2005Status:Defferedand Re-indexedDeferDate:13.07.2005Decision :The committee considered this case for fixation <strong>of</strong> I/O <strong>No</strong>rms for the export product Carbidopa under Para 4.7 <strong>of</strong> HBP as peragenda.The representative <strong>of</strong> PI Division requested for sending him a copy <strong>of</strong> the application <strong>of</strong> the firm again. The Committee decided to send onemore copy <strong>of</strong> application to PI Division and deferred the case for four weeks.23Case <strong>No</strong>.:3/42/82-ALC1/2004HQ File :01/82/050/01749/AM05/Party Name:EMAMI LIMITED,RLA File :02/24/040/00244/AM05/Meet <strong>No</strong>/Date:<strong>10</strong>/82-ALC1/200508.06.2005Lic.<strong>No</strong>/Date:02<strong>10</strong>07421620.01.2005Status:CaseRejectedDefer Date:Decision :The committee considered this case for fixation <strong>of</strong> I/O <strong>No</strong>rms for the export product Boroplus Skincare Cream under Para 4.7 <strong>of</strong>HBP as per agenda.The Committee noted that information had been called for from the firm vide letter <strong>dated</strong> 15.3.05 and that reply from the firm had not beenreceived. In the absence <strong>of</strong> the information called for from the firm, it was not possible for the Committee to work out the input outputnorms. The Committee accordingly decided to reject the case.RLA concerned may take further necessary action including cancellation <strong>of</strong> licence under Para 4.7 <strong>of</strong> HBP as per above decision <strong>of</strong> the
http://serverdgft:8<strong>10</strong>0/dgft/PrintServ?mode=0&id=2005-09-22%2013:01:27.500002<strong>10</strong> <strong>of</strong> 17 22.09.05 12:58 PMCommittee.24Case <strong>No</strong>.:51/41/82-ALC1/2004HQ File :01/82/050/01789/AM05/Party Name:MALLADI DRUGS &PHARMACEUTICALS LIMITEDRLA File :04/24/040/00269/AM05/Meet <strong>No</strong>/Date:<strong>10</strong>/82-ALC1/200508.06.2005Lic.<strong>No</strong>/Date:04<strong>10</strong>06674001.02.2005Status:CaseApprovedDefer Date:Decision :Withdrawn. This case has already been approved by the Advance Licensing Committee in its meeting <strong>No</strong>.8/06 held on 25.5.05.25Case <strong>No</strong>.:<strong>10</strong>/1/82-ALC1/2005HQ File :01/82/050/01805/AM05/Party Name:DIVIS LABORATORIES LTD.,RLA File :09/24/040/00340/AM05/Meet <strong>No</strong>/Date:<strong>10</strong>/82-ALC1/200508.06.2005Lic.<strong>No</strong>/Date:09<strong>10</strong>02173803.02.2005Status:Defferedand Re-indexedDeferDate:13.07.2005Decision :The Committee considered this case for fixation <strong>of</strong> I/O <strong>No</strong>rms for the export product 5-Nitro Isophthalic Acid Dimethyl Esterunder Para 4.7 <strong>of</strong> HBP as per agenda.The representative <strong>of</strong> PI Division sought another copy <strong>of</strong> the application <strong>of</strong> the firm. The Committee decided to send him the same and todefer the case for four weeks.26Case <strong>No</strong>.:11/1/82-ALC1/2005HQ File :01/82/050/01806/AM05/Party Name:DIVIS LABORATORIES LTD.,RLA File :09/24/040/00345/AM05/Meet <strong>No</strong>/Date:<strong>10</strong>/82-ALC1/200508.06.2005Lic.<strong>No</strong>/Date:09<strong>10</strong>02173903.02.2005Status:CaseApprovedDefer Date:Decision :Decision :The Committee considered this case for fixation <strong>of</strong> I/O norms for the export product Gabapentin under para 4.7 <strong>of</strong> HBPas per agenda.The Committee in light <strong>of</strong> the written comments <strong>of</strong> the Deptt. <strong>of</strong> C&PC forwarded vide their U.O.<strong>No</strong>. 35011/183/2004-PI-III <strong>dated</strong> 26.5.05decided to fix I/O <strong>No</strong>rms for the export product for the purpose <strong>of</strong> para 4.7 <strong>of</strong> HBP only as under:Export productGabapentin--------1kgImport items1. 1,1 Cyclohexane Diaceticacid-----2.65 kg2. IRA-67-----------0.65 kg3. Iso Propyl Alcohol---3.56 kgRLA concerned may take further necessary action under para 4.7 <strong>of</strong> HBP as per above decision <strong>of</strong> ALC.
http://serverdgft:8<strong>10</strong>0/dgft/PrintServ?mode=0&id=2005-09-22%2013:01:27.50000211 <strong>of</strong> 17 22.09.05 12:58 PMCase <strong>No</strong>.:12/1/82-ALC1/2005HQ File :01/82/050/01807/AM05/Party Name:DIVIS LABORATORIES LTD.,RLA File :09/24/040/00346/AM05/Meet <strong>No</strong>/Date:<strong>10</strong>/82-ALC1/200508.06.2005Lic.<strong>No</strong>/Date:09<strong>10</strong>02174003.02.2005Status:CaseApprovedDefer Date:Decision :The Committee considered this case for fixation <strong>of</strong> I/O norms for the export product Bal 5661 under para 4.7 <strong>of</strong> HBP as peragenda.The Committee in light <strong>of</strong> the written comments <strong>of</strong> the Deptt. <strong>of</strong> C&PC forwarded vide their U.O.<strong>No</strong>. 35011/183/2004-PI-III <strong>dated</strong> 26.5.05decided to fix I/O <strong>No</strong>rms for the export product for the purpose <strong>of</strong> para 4.7 <strong>of</strong> HBP only as under:27Export product1. (Z)-[5-amino-[1,2,4]Thiadiazo-3-yl]-Trityl oxyimino-thiacetic acid-S-benzothiazol-2-yl-ester(Bal 5661)---------1kgImport items1. Malononitrile-----<strong>10</strong>.78 kg2. Methanol--------20.64 kg3. Methyl Tertiary Butyl ether-----26.92 kg4. Hydroxylamine Hcl-----4.00 kg5. Acetonitrile--------9.27 kg6. Potassium thiocyanate----6.09 kgRLA concerned may take further necessary action under para 4.7 <strong>of</strong> HBP as per above decision <strong>of</strong> ALC.28Case <strong>No</strong>.:26/1/82-ALC1/2005HQ File :01/82/050/01803/AM05/Party Name:HETERO DRUGS LIMITED,RLA File :09/24/040/00344/AM05/Meet <strong>No</strong>/Date:<strong>10</strong>/82-ALC1/200508.06.2005Lic.<strong>No</strong>/Date:09<strong>10</strong>02173603.02.2005Status:Defferedand Re-indexedDeferDate:13.07.2005Decision :The Committee considered this case for fixation <strong>of</strong> I/O norms for the export product Cefpodoxime Proxetil under para 4.7 <strong>of</strong> HBPas per agenda.The representative <strong>of</strong> PI Division stated that the case is under examination and sought deferment. The Committee accordingly deferred thecase for four weeks.
http://serverdgft:8<strong>10</strong>0/dgft/PrintServ?mode=0&id=2005-09-22%2013:01:27.50000212 <strong>of</strong> 17 22.09.05 12:58 PMCase <strong>No</strong>.:27/1/82-ALC1/2005HQ File :01/82/050/01808/AM05/Party Name:M/S.ENAL DRUGS PVT LTD.,RLA File :09/24/040/00334/AM05/Meet <strong>No</strong>/Date:<strong>10</strong>/82-ALC1/200508.06.2005Lic.<strong>No</strong>/Date:09<strong>10</strong>02174<strong>10</strong>3.02.2005Status:CaseApprovedDefer Date:Decision :The Committee considered this case for fixation <strong>of</strong> I/O norms for the export product under para 4.7 <strong>of</strong> HBP as per agenda.The Committee in light <strong>of</strong> the written comments <strong>of</strong> the Deptt. <strong>of</strong> C&PC(PI Division forwarded vide their U.O.<strong>No</strong>. 35011/17/2005 PI-III<strong>dated</strong> 12.5.05 decided to fix I/O <strong>No</strong>rms for the export product for the purpose <strong>of</strong> para 4.7 <strong>of</strong> HBP only as under:29Export product2,3 Dimethyl-4-Nitro Pyridine N-Oxide--------1kgImport items1. 2,3 Lutidine----------------0.85 kg2. Methylene Chloride (MDC)----1.175 kg3. Iso Propyl Alcohol-----0.46 kgRLA concerned may take further necessary action under para 4.7 <strong>of</strong> HBP as per above decision <strong>of</strong> ALC.30Case <strong>No</strong>.:29/1/82-ALC1/2005HQ File :01/82/050/01859/AM05/Party Name:HETERO LABS LTD.RLA File :09/24/040/00352/AM05/Meet <strong>No</strong>/Date:<strong>10</strong>/82-ALC1/200508.06.2005Lic.<strong>No</strong>/Date:09<strong>10</strong>02181708.02.2005Status:Defferedand Re-indexedDeferDate:13.07.2005Decision :The Committee considered this case for fixation <strong>of</strong> I/O norms for the export product Cilostazol under para 4.7 <strong>of</strong> HBP as peragenda.The representative <strong>of</strong> PI Division stated that the case is under examination. The Committee accordingly decided to defer the case for fourweeks.31Case <strong>No</strong>.:56/41/82-ALC1/2004HQ File :01/82/050/01846/AM05/Party Name:MALLADI DRUGS &PHARMACEUTICALS LIMITEDRLA File :04/24/040/00263/AM05/Meet <strong>No</strong>/Date:<strong>10</strong>/82-ALC1/200508.06.2005Lic.<strong>No</strong>/Date:04<strong>10</strong>06700208.02.2005Status:CaseApprovedDefer Date:Decision :The Committee considered this case for fixation <strong>of</strong> I/O norms for the export product under para 4.7 <strong>of</strong> HBP as per agenda.The Committee in light <strong>of</strong> the written comments <strong>of</strong> the Deptt. <strong>of</strong> C&PC forwarded vide their U.O.<strong>No</strong>. 35011/21/2005-PI-III <strong>dated</strong> 12.5.05
http://serverdgft:8<strong>10</strong>0/dgft/PrintServ?mode=0&id=2005-09-22%2013:01:27.50000213 <strong>of</strong> 17 22.09.05 12:58 PMdecided to fix I/O <strong>No</strong>rms for the export product for the purpose <strong>of</strong> para 4.7 <strong>of</strong> HBP only as under:Export product1(4-Methylphenyl)-2Pyridyl-3-Pyrrolidinolpropan-1-OL(Carbinol)----1kgImport items1. 4-Methyl Acetophenone--------1.04 kg2. Pyrrolidine-------0.60 kg3. Iso Propyl Alcohol---------1.26 kg4. Lithium-------0.<strong>10</strong>0 kg5. 2-Bromo Pyridine-------1.12 kg6. Toluene-----0.728 kgRLA concerned may take further necessary action under para 4.7 <strong>of</strong> HBP as per above decision <strong>of</strong> ALC.32Case <strong>No</strong>.:31/1/82-ALC1/2005HQ File :01/82/050/01876/AM05/Party Name:ESTEEM INDUSTRIES PVT.LTD,RLA File :17/24/040/00042/AM05/Meet <strong>No</strong>/Date:<strong>10</strong>/82-ALC1/200508.06.2005Lic.<strong>No</strong>/Date:17<strong>10</strong>000978<strong>10</strong>.02.2005Status:Defferedand Re-indexedDeferDate:13.07.2005Decision :The committee considered this case for fixation <strong>of</strong> I/O <strong>No</strong>rms for the export product Esteem-143 AG under Para 4.7 <strong>of</strong> HBP asper agenda.The Committee noted that details <strong>of</strong> manufacturing process had been called for from the firm vide letter <strong>dated</strong> 30.5.05. The Committeedecided to await reply from the firm and deferred the case for four weeksCase <strong>No</strong>.:7/44/82-ALC1/2004HQ File :01/82/050/01895/AM05/Party Name:MOREPEN LABORATORIESLTD.,RLA File :05/24/040/00588/AM05/Meet <strong>No</strong>/Date:<strong>10</strong>/82-ALC1/200508.06.2005Lic.<strong>No</strong>/Date:05<strong>10</strong>15098714.02.2005Status:CaseApprovedDefer Date:Decision :The Committee considered this case for fixation <strong>of</strong> I/O norms for the export product under para 4.7 <strong>of</strong> HBP as per agenda.33The Committee in light <strong>of</strong> the written comments <strong>of</strong> the Deptt. <strong>of</strong> C&PC forwarded vide their U.O.<strong>No</strong>. 35011/249/2004-PI-III <strong>dated</strong> 12.5.05decided to fix I/O <strong>No</strong>rms for the export product for the purpose <strong>of</strong> para 4.7 <strong>of</strong> HBP only as under:Export productSultamicillin Tosylate--------1kg
http://serverdgft:8<strong>10</strong>0/dgft/PrintServ?mode=0&id=2005-09-22%2013:01:27.50000214 <strong>of</strong> 17 22.09.05 12:58 PMImport items1. Sulbactam Acid--------0.99 kg2. Di chloro Methane/Methylene Chloride-------2.63 kg3. Ampicillin Anhydrous-------0.80 kgRLA concerned may take further necessary action under para 4.7 <strong>of</strong> HBP as per above decision <strong>of</strong> ALC34Case <strong>No</strong>.:11/46/82-ALC1/2004HQ File :01/82/050/01905/AM05/Party Name:INDOKEM LTD.[FORMERLYKNOWN AS KHATAU JUNKER LTD.]RLA File :03/24/040/02<strong>10</strong>7/AM05/Meet <strong>No</strong>/Date:<strong>10</strong>/82-ALC1/200508.06.2005Lic.<strong>No</strong>/Date:03<strong>10</strong>31711316.02.2005Status:Defferedand Re-indexedDeferDate:13.07.2005Decision :The committee considered this case for fixation <strong>of</strong> I/O <strong>No</strong>rms for the export product Indonon Dark Blue G CDP (Vat Blue 16CDP) under Para 4.7 <strong>of</strong> HBP as per agenda.The representative <strong>of</strong> Chemical Division stated that the case is under examination. The Committee accordingly, decided to defer the case for4 weeks.35Case <strong>No</strong>.:13/1/82-ALC1/2005HQ File :01/82/050/01943/AM05/Party Name:MRF LIMITEDRLA File :04/24/040/00262/AM05/Meet <strong>No</strong>/Date:<strong>10</strong>/82-ALC1/200508.06.2005Lic.<strong>No</strong>/Date:04<strong>10</strong>06774525.02.2005Status:Defferedand Re-indexedDeferDate:13.07.2005Decision :The committee considered this case for fixation <strong>of</strong> I/O <strong>No</strong>rms for the export product Steel Radial Passenger Car Tyre (Tube Type)under Para 4.7 <strong>of</strong> HBP as per agenda.The Committee noted that information had been called for from the firm vide letter dt. 19.5.05. The Committee accordingly, decided toawait the same and deferred the case for 4 weeks.36Case <strong>No</strong>.:44/1/82-ALC1/2005HQ File :01/82/050/01981/AM05/Party Name:IPCA LABORATORIESLIMITEDRLA File :03/24/040/02583/AM05/Meet <strong>No</strong>/Date:<strong>10</strong>/82-ALC1/200508.06.2005Lic.<strong>No</strong>/Date:03<strong>10</strong>32033507.03.2005Status:Defferedand Re-indexedDeferDate:13.07.2005Decision :The committee considered this case for fixation <strong>of</strong> I/O <strong>No</strong>rms for the export product Hydroxy Chloroquine Sulphate under Para4.7 <strong>of</strong> HBP as per agenda. The representative <strong>of</strong> PI Division stated that the case is under examination. The Committee accordingly deferredthe case for 4 weeks.
http://serverdgft:8<strong>10</strong>0/dgft/PrintServ?mode=0&id=2005-09-22%2013:01:27.50000215 <strong>of</strong> 17 22.09.05 12:58 PM37Case <strong>No</strong>.:14/1/82-ALC1/2005HQ File :01/82/050/01983/AM05/Party Name:GPL POLYFILS (A DIV.GANESH POLYTEX LTD.)RLA File :06/80/040/00<strong>10</strong>2/AM05/Meet <strong>No</strong>/Date:<strong>10</strong>/82-ALC1/200508.06.2005Lic.<strong>No</strong>/Date:06<strong>10</strong>00891709.03.2005Status:Defferedand Re-indexedDeferDate:13.07.2005Decision :The committee considered this case for fixation <strong>of</strong> I/O <strong>No</strong>rms for the export product Polyester Staple Fibre under Para 4.7 <strong>of</strong> HBPas per agenda. The representative <strong>of</strong> PC Division stated that the case is under examination. The Committee accordingly deferred the case for4 weeks.38Case <strong>No</strong>.:46/1/82-ALC1/2005HQ File :01/82/050/02017/AM05/Party Name:INDIA GLYCOLS LTDRLA File :05/88/040/00258/AM05/Meet <strong>No</strong>/Date:<strong>10</strong>/82-ALC1/200508.06.2005Lic.<strong>No</strong>/Date:05<strong>10</strong>15314014.03.2005Status:Defferedand Re-indexedDeferDate:13.07.2005Decision :The committee considered this case for fixation <strong>of</strong> I/O <strong>No</strong>rms for the export product Polyethylene Glycol -400 (Polymeg-400)under Para 4.7 <strong>of</strong> HBP as per agenda. The representative <strong>of</strong> PC Division stated that the case is under examination. The Committeeaccordingly deferred the case for 4 weeks.39Case <strong>No</strong>.:1/<strong>10</strong>/82-ALC1/2005HQ File :01/82/050/02096/AM05/Party Name:BASE METALCHLORINATIONS (P) LTDRLA File :34/24/040/00201/AM05/Meet <strong>No</strong>/Date:<strong>10</strong>/82-ALC1/200508.06.2005Lic.<strong>No</strong>/Date:34<strong>10</strong>013<strong>10</strong>129.03.2005Status:CaseApprovedDefer Date:Decision :The committee considered this case for fixation <strong>of</strong> I/O <strong>No</strong>rms for the export product Calcium Butyrate under Para 4.7 <strong>of</strong> HBP asper agenda. The Committee in light <strong>of</strong> the written comments <strong>of</strong> the Deptt. <strong>of</strong> C&PC forwarded vide their U.O.<strong>No</strong>. 140<strong>10</strong>/147/2004/Ch-II<strong>dated</strong> 28.<strong>10</strong>.04 decided to fix I/O <strong>No</strong>rms for the export product for the purpose <strong>of</strong> para 4.7 <strong>of</strong> HBP only as under:Export productCalcium Butyrate--------1 kgImport itemsButyric Acid--------0.788 kgRLA concerned may take further necessary action under para 4.7 <strong>of</strong> HBP as per above decision <strong>of</strong> ALC.
http://serverdgft:8<strong>10</strong>0/dgft/PrintServ?mode=0&id=2005-09-22%2013:01:27.50000216 <strong>of</strong> 17 22.09.05 12:58 PM40Case <strong>No</strong>.:2/6/82-ALC1/2005HQ File :01/82/050/00030/AM06/Party Name:CONCORD BIOTECH LTDRLA File :08/80/040/00530/AM05/Meet <strong>No</strong>/Date:<strong>10</strong>/82-ALC1/200508.06.2005Lic.<strong>No</strong>/Date:08<strong>10</strong>04726907.04.2005Status:Defferedand Re-indexedDeferDate:13.07.2005Decision :The committee considered this case for fixation <strong>of</strong> I/O <strong>No</strong>rms for the export product Pravastatin Sodium EP under Para 4.7 <strong>of</strong>HBP as per agenda. The Committee noted that the case had been referred to DBT and decided to await reply. The Committee accordinglydeferred the case for 4 weeks.41Case <strong>No</strong>.:3/<strong>10</strong>/82-ALC1/2005HQ File :01/82/050/00051/AM06/Party Name:CIBA SPECIALTY CHEMICALS(INDIA) LTD.RLA File :17/24/040/00003/AM06/Meet <strong>No</strong>/Date:<strong>10</strong>/82-ALC1/200508.06.2005Lic.<strong>No</strong>/Date:17<strong>10</strong>00<strong>10</strong>2615.04.2005Status:Defferedand Re-indexedDeferDate:13.07.2005Decision :The committee considered this case for fixation <strong>of</strong> I/O <strong>No</strong>rms for the export product Uvitex OB under Para 4.7 <strong>of</strong> HBP as peragenda. The representative <strong>of</strong> Chemical Division stated that the case is under examination. The Committee accordingly deferred the case for4 weeks.42Case <strong>No</strong>.:4/<strong>10</strong>/82-ALC1/2005HQ File :01/82/050/00069/AM06/Party Name:DOCTORSORGANICCHEMICALS LTDRLA File :26/80/040/00003/AM06/Meet <strong>No</strong>/Date:<strong>10</strong>/82-ALC1/200508.06.2005Lic.<strong>No</strong>/Date:26<strong>10</strong>00467627.04.2005Status:Defferedand Re-indexedDeferDate:13.07.2005Decision :The committee considered this case for fixation <strong>of</strong> I/O <strong>No</strong>rms for the export product Mefenamic Acid under Para 4.7 <strong>of</strong> HBP asper agenda. The representative <strong>of</strong> PI Division stated that the case is under examination. The Committee accordingly deferred the case for 4weeks.43Case <strong>No</strong>.:2/<strong>10</strong>/82-ALC1/2005HQ File :01/82/050/00070/AM06/Party Name:BASE METALCHLORINATIONS (P) LTDRLA File :34/24/040/00017/AM06/Meet <strong>No</strong>/Date:<strong>10</strong>/82-ALC1/200508.06.2005Lic.<strong>No</strong>/Date:34<strong>10</strong>01326928.04.2005Status:CaseApprovedDefer Date:Decision :The committee considered this case for fixation <strong>of</strong> I/O <strong>No</strong>rms for the export product Calcium Butyrate under Para 4.7 <strong>of</strong> HBP asper agenda. The Committee in light <strong>of</strong> the written comments <strong>of</strong> the Deptt. <strong>of</strong> C&PC forwarded vide their U.O.<strong>No</strong>. 140<strong>10</strong>/147/2004/Ch-II<strong>dated</strong> 28.<strong>10</strong>.04 decided to fix I/O <strong>No</strong>rms for the export product for the purpose <strong>of</strong> para 4.7 <strong>of</strong> HBP only as under:Export productCalcium Butyrate--------1 kgImport itemsButyric Acid--------0.788 kg
http://serverdgft:8<strong>10</strong>0/dgft/PrintServ?mode=0&id=2005-09-22%2013:01:27.50000217 <strong>of</strong> 17 22.09.05 12:58 PMRLA concerned may take further necessary action under para 4.7 <strong>of</strong> HBP as per above decision <strong>of</strong> ALC.Date:22.09.2005Place:New DelhiDevi Ram MeenaLicence Assistant/ Console Operator