Zeiss has a microscope for - Mines Magazine - Colorado School of ...
Zeiss has a microscope for - Mines Magazine - Colorado School of ...
Zeiss has a microscope for - Mines Magazine - Colorado School of ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
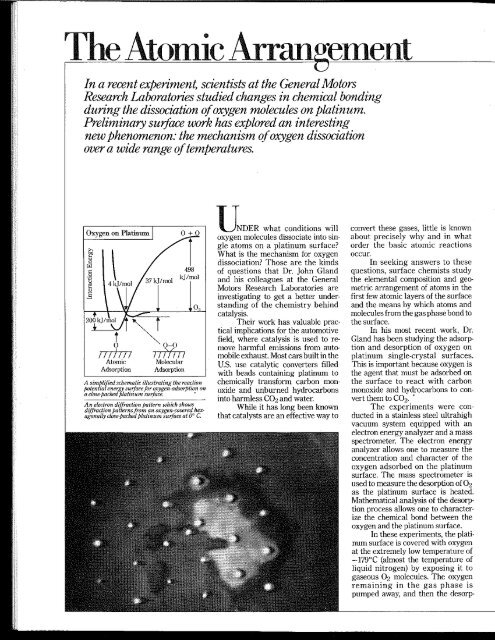
In a recent experiment, scientists at the General MotorsResearch Laboratories studied changes in chemical bondingduring the dissociation <strong>of</strong> oxygen molecules on platinum.Preliminary surface work <strong>has</strong> explored an interestingnew phenomenon: the mechanism <strong>of</strong> oxygen dissociationover a wide range <strong>of</strong> temperatures.Oxygen on Platinum 0+0OIII) i nAtomicAdsorption0-011 InfillMolecularAdsorptionA simplified scliematic illuslrating lite reactionpotential energy surface <strong>for</strong> oxygen-adsorption ona close-paclted platinum surface.An electron diffraction pattern whicli sliowsdiffraction patterns from an oxygen-covered hexagonallyclose-paclted platinum surface at 0° C.'NDER what conditions willoxygen molecules dissociate into singleatoms on a platinum surface?What is the mechanism <strong>for</strong> oxygendissociation? Those are the kinds<strong>of</strong> questions that Dr. John Glandand his colleagues at the GeneralMotors Research Laboratories areinvestigating to get a better understanding<strong>of</strong> the chemistry behindcatalysis.Their work <strong>has</strong> valuable practicalimplications <strong>for</strong> the automotivefield, where catalysis is used to removeharmful emissions from automobileexhaust. Most cars built in theU.S. use catalytic converters filledwith beads containing platinum tochemically trans<strong>for</strong>m carbon monoxideand unburned hydrocarbonsinto harmless CO2 and water.While it <strong>has</strong> long been knownthat catalysts are an effective way toconvert these gases, little is knownabout precisely why and in whatorder the basic atomic reactionsoccur.In seeking answers to thesequestions, surface chemists studythe elemental composition and geometricarrangement <strong>of</strong> atoms in thefirst few atomic layers <strong>of</strong> the surfaceand the means by which atoms andmolecules from the gas p<strong>has</strong>e bond tothe surface.In his most recent work, Dr.Gland <strong>has</strong> been studying the adsorptionand desorption <strong>of</strong> oxygen onplatinum single-crystal surfaces.Hiis is important because oxygen isthe agent that must be adsorbed onthe surface to react with carbonmonoxide and hydrocarbons to convertthem to CO2.The experiments were conductedin a stainless steel ultrahighvacuum system equipped with anelectron energy analyzer and a massspectrometer. The electron energyanalyzer allows one to measure theconcentration and character <strong>of</strong> theoxygen adsorbed on the platinumsurface. The mass spectrometer isused to measure the desorption <strong>of</strong> O2as the platinum surface is heated.Mathematical analysis <strong>of</strong> the desorptionprocess allows one to characterizethe chemical bond between theoxygen and the platinum surface.In these experiments, the platinumsurface is covered with oxygenat the extremely low temperature <strong>of</strong>—179°C (almost the temperature <strong>of</strong>liquid nitrogen) by exposing it togaseous O2 molecules. The oxygenremaining in the gas p<strong>has</strong>e ispumped away, and then the desorption<strong>of</strong> oxygen from the surface isobserved as the- platinum crystal isgradually heated to 1000°C.The oxygen was found to desorbfrom the surface in twodistinctly different temperatureregimes—part at —-125°C and therest at about 425°C. By using theoxygen-18 isotope, it was establishedthat the low temperature desorptionrepresents oxygen that wasadsorbed on the surface in a molecular<strong>for</strong>m whiie the higher temperaturedesorption corresponds tooxygen adsorbed in the atomic <strong>for</strong>m.From an analysis <strong>of</strong> the desorptionprocess, it was possible to establishthe complete energetics. Oxygenmolecules from the gas p<strong>has</strong>e strikethe surface and are weakly bound (37kJ/mol).The adsorbed oxygen moleculecan either desorb into the gasp<strong>has</strong>e (37 kj/mol) or dissociate intoatoms (33 kJ/mo!). The atoms arebonded very strongly (200 kJ/mol) tothe surface..ROM the desorption analysis,it was also possible to deduce themechanism <strong>for</strong> the dissociation process.The interesting conclusion thatresults is that the <strong>for</strong>mation <strong>of</strong> 0atoms on platinum is a two-step process—oxygenis adsorbed in a molecularstate and then dissociates to <strong>for</strong>matoms.The GM scientists were mostinterested in learning how this adsorbedmolecular species is bondedto the platinum surface. Fortunately,another technique was available todetermine the bonding. The techniqueis called electron energy-lossspectroscopy and is quite new—thereare only six or seven such instrumentsin the world. The measurementsnot only confirmed theexistence <strong>of</strong> the adsorbed molecularoxygen but showed that it was boundby the transfer <strong>of</strong> two electronsfrom the platinum surface into theantibonding it^ orbitals <strong>of</strong> oxygen."This was most exciting" said Dr.Gland, "because this is the first timethat this type <strong>of</strong> oxygen bond <strong>has</strong>been observed on a metal surface."We're getting closer andcloser to a more specific understanding<strong>of</strong> catalysis," says Dr. Gland."The more we learn about simplechemical systems, the better we'll beable to control more complicated systems.That <strong>has</strong> excellent implications<strong>for</strong> protecting the environment."^pjjgDr. John Gland,AT AIV years old, is a; X^t^-^r-ivTT-v Senior Research Sci-OliirilJNID entist in surfaceX H E chemistry at thex|yr\r>iT General Motors Re-Wv/IaJV search Laboratories.He heads a group <strong>of</strong> 7 investigators, 4with Ph.D.s, all involved in work relatingto the basic surface chemistry<strong>of</strong> catalysis.A graduate <strong>of</strong> WhittenbergUniversity in Ohio, Dr. Gland receivedhis Ph.D. in physical chemistryat the University<strong>of</strong> Cali<strong>for</strong>nia,Berkeley, in 1973and joined theGeneral Motorsstaff that year.Dr. Glandcomments: "Icame to GM Labsbecause I wantedto get in on theground floor <strong>of</strong>an exciting newfield. The atmospherehere is very open, with lots <strong>of</strong>cross-pollination among departments.With several hundred peoplewith Ph.D.s here, we've got a lot <strong>of</strong>human resources to draw on in allthe basic sciences."Typically, management definesa broad problem, then we're freeto tackle the solution in any way wechoose. They give us the freedom,equipment and support to get the jobdone correctly."In addition to his research. Dr.Gland enjoys backpacking inWyoming and in the Sierra NevadaMountains in Cali<strong>for</strong>nia.