R T / Θvib - Chem.hope.edu
R T / Θvib - Chem.hope.edu
R T / Θvib - Chem.hope.edu
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
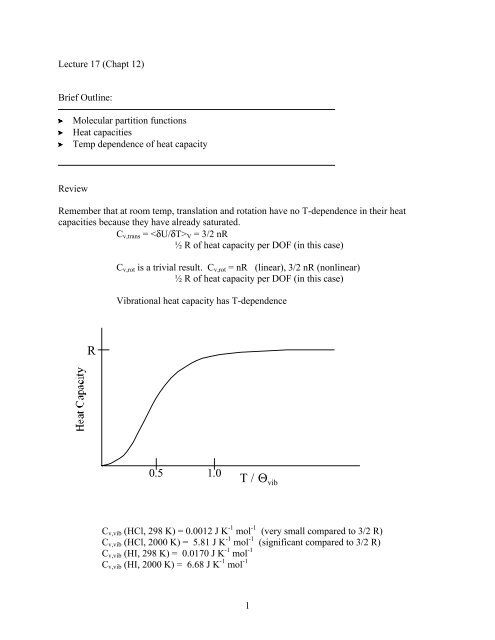
Lecture 17 (Chapt 12)Brief Outline:Molecular partition functionsHeat capacitiesTemp dependence of heat capacityReviewRemember that at room temp, translation and rotation have no T-dependence in their heatcapacities because they have already saturated.C v,trans = V = 3/2 nR½ R of heat capacity per DOF (in this case)C v,rot is a trivial result. C v,rot = nR (linear), 3/2 nR (nonlinear)½ R of heat capacity per DOF (in this case)Vibrational heat capacity has T-dependenceR0.5 1.0T / Θ vibC v,vib (HCl, 298 K) = 0.0012 J K -1 mol -1 (very small compared to 3/2 R)C v,vib (HCl, 2000 K) = 5.81 J K -1 mol -1 (significant compared to 3/2 R)C v,vib (HI, 298 K) = 0.0170 J K -1 mol -1C v,vib (HI, 2000 K) = 6.68 J K -1 mol -11
C v,vib (HI or HCl, infinite temp) = 8.31 J K -1 mol -1 (the maximum contribution tothe heat capacity for each vibrational mode is R) High temperature limit isT > Θ(indicate on figure)We can see here that the vibrational contribution to the heat capacity depends on thetemperature and bond strength of the molecule (frequencies of its vibrationalmodes). This contribution is the primary reason why heat capacity does, ingeneral, depend on temperature for polyatomic molecules.Finally for electronic the energy spacings are huge, so the ground state is the only one thatcontributes, even at very high temperatures.=−βεjq g eelecHCl: 298 Kq HCl∑levelsjelec= 1 (energy of lowest excited state is much higher than kT at 298 K)For most atoms and molecules, the electronic degree of freedom does not significantlycontribute to the average energy or to the heat capacity = 0 or U elec = 0 and C v,elec = 0SummaryOK, so let’s go back and summarize. For HCl the partition function is (ask for values?)huge for translational (10 29 )a handful for rotational (20)just over 1 for vibrational (1.0000007)and exactly 1 for electronic (1)We can now sum up the total heat capacity for a molecule like HCl at 298 KC v,298 K = C v,trans + C v,rot + C v,vib + C v,elec = 3/2 R + R + C v,vib + C v,elec= 12.46 + 8.31 + 0.0012 + 0 J K -1 mol -1 = 20.78 J K -1 mol -1The experimental value at 298 K is 20.81 J K -1 mol -1 .2
At 2000 K, HCl has a higher heat capacity because of? it has more vibrational modes availableto store energy:C v,2000 K = 26.59 J K -1 mol -1Total heat capacity is just sum of all component heat capacitiesIndividual contributions (have students fill in table):Translation Rotation Vibration ElectronicDiatomic (linear) 3/2 R R R*(0 ö 3N-5) 0polyatomic (nonlinear) 3/2 R 3/2 R R*(0 ö 3N-6) 0At long last on to Chapt 12 and we’ll finish this today and talk about homework – stop me at10:15.This will be quick because I’m not a big fan of Chapt 12, but it does introduce one veryimportant concept – density of states or DOS.This is really just another way to think about degeneracy when we are in a system were energy isapproximately continuous… such as? translation (in 3-D).εtrans, n2⎛2 2h⎜n nx y n+ +2 28m⎝ a b c2= z2⎞⎟⎠to make it a little simpler, set a = b = cεtrans,n2h=8ma22 2 2( n + n + n )xyzlowest energy state is? (n x ,n y ,n z ) = 1,1,1E = 3 unitsnext state is? 1,1,2 or 1,2,1 or 2,1,1 E = 6 unitsYou can imagine when we get up to a reasonably high energy there will be many possiblecombinations. Let’s see if we can figure this out quantitatively…Anyone recognize another way we might representIf we switch to spherical coordinates, this is R 2n + n + n ?2x2y2z3
εtrans , r=2h8ma2R2Let’s try to think about degeneracy now. Picture our sphere... all the combinations ofx,y,z values that reside on the surface of the sphere give the same energy. Let’s sketchthis out in two dimensions. (sketch 1 quadrant with dots at integer x,y. then draw anannulus (e.g. area of r + dr)).So, let’s call M the number of integers contained in our sphere.1 ⎛ 4π3 ⎞M = ⎜ R ⎟8 ⎝ 3 ⎠The number of integers in our little shell from r to r + dr (or ε to ε + dε) is:dMW ( ε ) dε= M ( ε + dε) − M ( ε ) = dεdεIf we take the energy equation we had earlier and solve for R we can substitute into the Mequation.2⎛ 8maε ⎞R =⎜2⎟⎝ h ⎠12Mπ ⎛ 8mε⎞= ⎜2⎟6 ⎝ h ⎠32V(if a 3 = V)322Wπ ⎛ 8m⎞⎜2⎟4 ⎝ h ⎠1( ε ) dε= VεdεW is the density of states.Let’s go one step further and take a look at the probability of occupying a certain state−εiβepi= if we rewrite this in terms of levels rather than statesqp( ε )W=( ε )eq−εβW increases rapidly with energy, and the Boltzmann factor decreases rapidly with energy.The result is Figure 12.5 in your book.4
OK, let’s talk a few minutes about homework for this long weekend.Because we’ve been behind in lecture we don’t have much new homework material.. I’lltack on what we’ve got to the next homework set. For this weekend then, I want you toget ready for next week’s seminar.I was going to give you one of her recent, short papers to read, but it is pretty steep. I’veput it on chemboard so you can take a look at it. You should be able to do well with theintroduction and Figure 1 and a little tidbit here and there. So, please take a look at it.However, the real requirement is to look up a few things. When Dr. Lester is here we aregoing to chat about where her work fits into the whole atmospheric ozone picture andthen a few specific things about how her research works. This should help you get a lotmore out of her seminar that afternoon.So, to get ready for next Friday’s lecture I want you to look up a few things that we willbe chatting about:1) What role do the NO x molecules play in ozone depletion?2) What is an overtone transition (with regard to vibrational transitions)?3) What is the Heisenberg uncertainty principle?5