QUESTIONSHEET 1 E/Z (CIS/TRANS) ISOMERISM
QUESTIONSHEET 1 E/Z (CIS/TRANS) ISOMERISM
QUESTIONSHEET 1 E/Z (CIS/TRANS) ISOMERISM
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
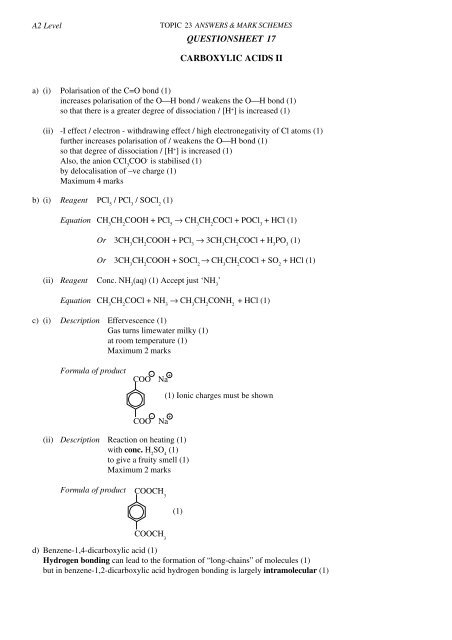
A2 LevelTOPIC 23 ANSWERS & MARK SCHEMES<strong>QUESTIONSHEET</strong> 17CARBOXYLIC ACIDS IIa) (i) Polarisation of the C=O bond (1)increases polarisation of the O⎯H bond / weakens the O⎯H bond (1)so that there is a greater degree of dissociation / [H + ] is increased (1)(ii) -I effect / electron - withdrawing effect / high electronegativity of Cl atoms (1)further increases polarisation of / weakens the O⎯H bond (1)so that degree of dissociation / [H + ] is increased (1)Also, the anion CCl 3COO - is stabilised (1)by delocalisation of –ve charge (1)Maximum 4 marksb) (i) Reagent PCl 5/ PCl 3/ SOCl 2(1)Equation CH 3CH 2COOH + PCl 5→ CH 3CH 2COCl + POCl 3+ HCl (1)Or 3CH 3CH 2COOH + PCl 3→ 3CH 3CH 2COCl + H 3PO 3(1)Or 3CH 3CH 2COOH + SOCl 2→ CH 3CH 2COCl + SO 2+ HCl (1)(ii) Reagent Conc. NH 3(aq) (1) Accept just ‘NH 3’Equation CH 3CH 2COCl + NH 3→ CH 3CH 2CONH 2+ HCl (1)c) (i) Description Effervescence (1)Gas turns limewater milky (1)at room temperature (1)Maximum 2 marksFormula of product-COO Na+(1) Ionic charges must be shownCOO Na(ii) Description Reaction on heating (1)with conc. H 2SO 4(1)to give a fruity smell (1)Maximum 2 marks-+Formula of productCOOCH 3COOCH 3(1)d) Benzene-1,4-dicarboxylic acid (1)Hydrogen bonding can lead to the formation of “long-chains” of molecules (1)but in benzene-1,2-dicarboxylic acid hydrogen bonding is largely intramolecular (1)