QUESTIONSHEET 1 E/Z (CIS/TRANS) ISOMERISM
QUESTIONSHEET 1 E/Z (CIS/TRANS) ISOMERISM
QUESTIONSHEET 1 E/Z (CIS/TRANS) ISOMERISM
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
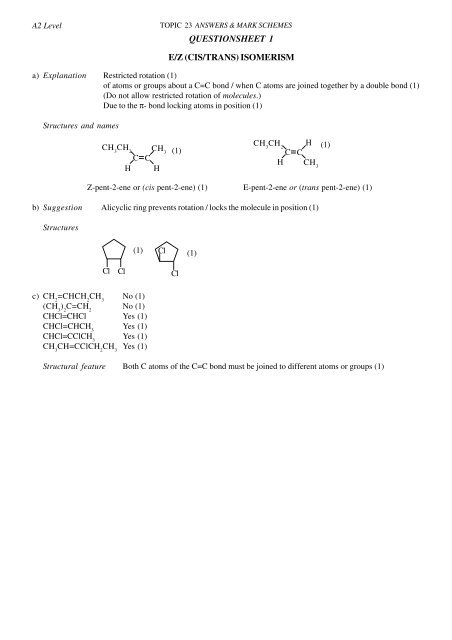
A2 LevelTOPIC 23 ANSWERS & MARK SCHEMES<strong>QUESTIONSHEET</strong> 1E/Z (<strong>CIS</strong>/<strong>TRANS</strong>) <strong>ISOMERISM</strong>a) Explanation Restricted rotation (1)of atoms or groups about a C=C bond / when C atoms are joined together by a double bond (1)(Do not allow restricted rotation of molecules.)Due to the π- bond locking atoms in position (1)Structures and namesCH 3CH 2CHCH 3CH(1)CH 3CH 2H (1)C CH CH 3Z-pent-2-ene or (cis pent-2-ene) (1) E-pent-2-ene or (trans pent-2-ene) (1)b) Suggestion Alicyclic ring prevents rotation / locks the molecule in position (1)Structures(1)Cl(1)ClClClc) CH 2=CHCH 2CH 3No (1)(CH 3) 2C=CH 2No (1)CHCl=CHCl Yes (1)CHCl=CHCH 3Yes (1)CHCl=CClCH 3Yes (1)CH 3CH=CClCH 2CH 3Yes (1)Structural feature Both C atoms of the C=C bond must be joined to different atoms or groups (1)
A2 LevelTOPIC 23 ANSWERS & MARK SCHEMES<strong>QUESTIONSHEET</strong> 3TEST QUESTION ON <strong>ISOMERISM</strong>a) (i) Skeletal isomerism CH 3CH 2CH 2CH 2OH / CH 3CH(OH)CH 2CH 3(1)and (CH 3) 2CHCH 2OH / (CH 3) 3COH (1)Positional isomerism CH 3CH 2CH 2CH 2OH (1) and CH 3CH(OH)CH 2CH 3(1)Or (CH 3) 2CHCH 2OH (1) and (CH 3) 3COH (1)Functional group isomerism Accept any of the C 4alcohols (1) and any of the C 4ethers (1)StereoisomerismHCH 3CC 2H 5OHCH 3(1) and CHOH(1)C 2H 5(ii) Butan-2-ol has a chiral centre / asymmetric C atom (1)∴ can exhibit optical isomerism / exist in two non-superimposable forms (1)but none of the compounds has a C = C bond (or other structural feature) which can cause restricted rotationof atoms (1)∴there is no possibility of geometrical isomerism / existence of cis and trans isomers (1)b) (i) Geometrical / cis-trans isomerism (1)(ii)CH 3CH 2C=N..CH 3NHC 6H 5(1)CH 3CH 2..C=NCH 3NH (1)C 6H 5(iii) Melting point / solubility (1)
A2 LevelTOPIC 23 ANSWERS & MARK SCHEMES<strong>QUESTIONSHEET</strong> 4STRUCTURE OF BENZENEa) (i) Platinum catalyst, room temperature / nickel catalyst, heat (1)(ii)(1)(iii) Since three double bonds are hydrogenated, ∆H = 3(-120) = -360 kJ mol -1 (1)(iv) Benzene is more stable than expected (1)by (360 – 208) = 152 kJ mol -1 (1)This is because benzene contains a delocalised system of electrons (1)b) (i) Delocalised Electrons are not restricted to “2-electron, 2-centre” bonds (1)but are dispersed / spread / mobile over a greater number of nuclear centres (1)UnsaturatedOne or more (carbon-carbon) bonds are multiple bonds / the compound will undergo an additionreaction with hydrogen (1)(ii)(1)(iii) Benzene does not undergo electrophilic addition reactions (1)XBenzene does not produce disubstituted isomers of the formXXandX(1)Electron-diffraction measurements show that all the carbon-carbon bonds are of equal length / intermediatebetween C-C and C=C (1)The enthalpy of hydrogenation of benzene is less exothermic than expected (1)The enthalpy of formation of benzene is less endothermic than expected (1)Maximum 3 marks
A2 LevelTOPIC 23 ANSWERS & MARK SCHEMES<strong>QUESTIONSHEET</strong> 5STRUCTURES OF CYLOALKENESa) Alkene I Cyclohexene (1)Alkene II 1,3-Cyclohexadiene (1)Alkene III 1,4-Cyclohexadiene (1)b)+ H 2→ (1)+ 2H 2→ (1)c) If the π-orbitals of II were completely localised / by comparison with cyclohexene (1)enthalpy of hydrogenation of II could be calculated as –119.6 x 2 = -239.2 kJ mol -1 (1)This is 7.5 kJ mol -1 different from the experimental value (1)∴II is energetically more stable than if it had two completely localised π-bonds (1)A slight degree of delocalisation of π-electrons must be occurring (1)Maximum 4 marksd) Shortest bonds will be a and c (1)Longest bonds will be d, e and f (1)b will be slightly shorter than d, e, and f (1)because of the slight degree of delocalisation (1)e) Estimate -239.2 kJ mol -1 (1)Reasons π-bonds are too far apart to overlap (1)No delocalisation of π-electrons (1)∴ no increase in stability (1)
A2 LevelTOPIC 23 ANSWERS & MARK SCHEMES<strong>QUESTIONSHEET</strong> 6BOND TYPE AND REACTIVITYa) (i) Electrophiles are attracted by electrons of the π-bond (1)The π-bond is weak / easily broken (1)(ii) C⎯H bonds are little polarised / C and H have similar electronegativity (1)For an alkane to react, strong σ-bonds would have to be broken (1)b) (i) Polarisation of the bond / C δ+ ⎯X δ- (1)due to the high electronegativity of halogens compared with carbon (1)(ii) RCl < RBr < RI (1)(iii) Bond strength decreases from C⎯Cl to C⎯I (1)hence a halide ion can leave more readily (1)This outweighs the decreasing strength of the –I effect / the lower electronegativity of the halogens (1)Maximum 2 marksc) (i) Nucleophilic reagents / nucleophiles (1)(ii) Bond polarisation C δ+ ⎯ O δ- / oxygen is much more electronegative than carbon (1)The π-bond is easily polarised (1)(iii) The π-bond is weak / easily broken (1)but the σ-bond is strong (1)(iv) They are repelled by the π-electrons of the C ⎯ C bond (1)(v) HCN would have to undergo heterolytic fission / dissociation (to H + and CN - ) (1)This is difficult because HCN is a very weak acid / because of the strength of the H⎯CN bond (1)
A2 LevelTOPIC 23 ANSWERS & MARK SCHEMES<strong>QUESTIONSHEET</strong> 7REACTION CONDITIONS AND PRODUCTa) (i) Free radical (½) substitution (½)(ii) Homolytic fission / homolysis (1)(iii) Stage 1Cl. Cl2Cl . (1)Stage 2.CH 3 CH2+ Cl . + HCl (1).CH2+ Cl 2CH 2Cl+ HCl (1)Stage 3.CH2 CH 2Cl+ Cl . (1)Or 2Cl . Cl 2(1)Or .2 CH2 CH 2CH 2(1)b) (i) CH 3 CH 3Cl+ Cl 2 + HCl (1)OrCH 3+ Cl 2CH 3+ HCl (1)(ii) Aluminium chloride / iron (III) chloride (1)Cl(iii)Electrophilic (½) substitution (½)(iv) Heterolytic fission / heterolysis (1)Aqueous NaOH (1)c) Example CH 3CH 2Br CH 3CH 2OH (1)Nucleophilic substitution (1)Ethanolic / alcoholic NaOH(1)CH 3CH 2Br CH 2= CH 2(1)Elimination (1)
A2 LevelTOPIC 23 ANSWERS & MARK SCHEMES<strong>QUESTIONSHEET</strong> 8NITRATION OF ARENESa) Reagents Concentrated nitric acid (1)and concentrated sulfuric acid (1)Conditions 50 - 60 °C (1)EquationNO 2+ HNO 3→ + H 2O (1)Or C 6H 6+ HNO 3→ C 6H 5NO 2+ H 2O (1)b) (i) Lower temperature / 30 - 40 °C (1)(ii) Use fuming nitric acid / more concentrated nitric acid (1)and a higher temperature (1)(iii) FormulaO 2NCH 3NO2NO 2(1)Use High explosive (1)c) (i) C 6H 5NO 2+ 6[H] → C 6H 5NH 2+ 2H 2O (1)NO 2NH 2Or + 6[H] → + 2H 2O (1)(ii)Reduction(1)(iii) Tin (1) and concentrated hydrochloric acid (1)(Accept HCl (aq) but not just ‘HCl’.)(iv) Primary aromatic amines form the basis of the azo dyestuffs industry (1)
A2 LevelTOPIC 23 ANSWERS & MARK SCHEMES<strong>QUESTIONSHEET</strong> 9HALOGENATION OF ARENESa) (i) Conditions Br 2in solution (in a named solvent) (1)Room temperature / 20 °C (1)BrBrEquation + Br 2→ (1)Or with molecular formulae in place of the structural formulae(ii) (Electrophilic) addition (1)b) (i) Conditions Br 2+ AlBr 3/ Al catalyst (1) (Allow AlCl 3)Room temperature / 20 °C (1)BrEquation + Br 2→ + HBr (1)Or with molecular formulae in place of the structural formulae(ii) (Electrophilic) substitution (1)c) π-electrons in cyclohexene are localised between two adjacent C atoms (1)π-electrons in benzene are delocalised over the six-membered ring (1)Therefore π-electrons in benzene attract bromine / the reagent / the electrophile less strongly (1)
A2 LevelTOPIC 23 ANSWERS & MARK SCHEMES<strong>QUESTIONSHEET</strong> 10ALKYLATION AND ACYLATION OF ARENESa) (i) Reagent Chloromethane / bromomethane / iodomethane (1)Conditions AlCl 3catalyst (1)Room temperature / 20 °C (1)Equation + CH 3Cl → + HCl (1)CH 3COCH 3Or C 6H 6+ CH 3Cl → C 6H 5CH 3+ HCl (1)(ii) It is difficult to prevent further alkylation / a dimethylbenzene or trimethylbenzene is formed readily (1)(iii) Type of reaction Oxidation (1)ReagentKMnO 4(½) + NaOH(aq) (½)Or K 2Cr 2O 7(½) + dil. H 2SO 4(½)Or dilute HNO 3(1)b) (i) Conditions AlCl 3catalyst (1)50 ºC / heat (1)Equation + CH 3COCl → + HCl (1)Or C 6H 6+ CH 3COCl → C 6H 5COCH 3+ HCl (1)Name of product Phenylethanone / acetophenone (1)(ii) Type of reaction Reduction (1)Reagent NaBH 4/ Zn + dil. H 2SO 4/ other named combination of metal and protic solvent (1)
A2 LevelTOPIC 23 ANSWERS & MARK SCHEMES<strong>QUESTIONSHEET</strong> 11PHENOLa) Identity of phenols I, II, & IV (1) (All must be correct)Basis of identification A phenol must have at least one –OH group attached directly to a benzene ring (1)b) (i) Identity of A Sodium phenoxide / sodium phenate / C 6H 5O Na (1)Identity of B Mainly phenol / (saturated) solution of water in phenol (1)(Allow just ‘phenol’)EquationsOHOr C 6H 5OH + NaOH → C 6H 5O Na + H 2O (1)-O Na+ NaOH → + H 2O (1)+- +-O Na + OH+ HCl → + NaCl (1)-+Or C 6H 5O Na + HCl → C 6H 5OH + NaCl (1)(ii) Reason 1 Lone pair of electrons on O (1)is drawn towards the benzene ring (1)O⎯H bond is weakened / increasingly polarised (1)so that H + is lost relatively easily (1)Maximum 3 marksReason 2 The phenoxide ion / C 6H 5O is stabilised (1)by delocalisation of –ve charge (1)over O and the benzene ring (1)-This encourages dissociation / ionisation / formation of C 6H 5O (1)No scope for delocalisation in an alkoxide ion / corresponding anion from an alcohol (1)Maximum 3 marksc) (i) White (½) precipitate (½)- +-(ii)OHOHBr Br+ 3Br 2→ + 3HBr (1)Br(iii) Lone pair of electrons on O (1)is drawn towards the benzene ring / occupies a p-orbital which overlaps the delocalised π-orbital of the ring (1)Hence electron density on the ring is increased (1)and the ring is activated towards electrophiles / electrophiles are more strongly attracted (1)Maximum 3 marksd) Antiseptics / disinfectants (1)
A2 LevelTOPIC 23 ANSWERS & MARK SCHEMES<strong>QUESTIONSHEET</strong> 12ALDEHYDES AND KETONESa) (i) C (½) Butanone / butan-2-one (½)(ii)A (½) Propanal (½) andB (½) 2-methylpropanal (½)(iii) C (½) Butanone (½)Allow if named in (i)b) (i) Silver mirror (1)(ii) propanoic acid (1)(iii) Fehlings (1) Red precipitate (1)or Potassium dichromate (VI) (1) orange to green (1)or potassium manganate(VII) (1) purple to colourless (1)c) (i) LiALH 4or NaBH 4(1)(ii) In dry ether (1) for LiALH 4or in solution in water or methanol for NaBH 4(1)
A2 LevelTOPIC 23 ANSWERS & MARK SCHEMES<strong>QUESTIONSHEET</strong> 13USE OF 2,4 - DINITROPHENYLHYDRAZINEa) C H OMoles 69.8/12 11.6/1 18.6/165.817 11.6 1.163 mol % (1)Ratio 5 10 1 (1)Empirical formula is C 5H 10O ; relative formula mass = 86 = relative molecular mass (1)therefore molecular formula is also C 5H 10O (1)b) Aldehydes: CH 3CH 2CH 2CH 2CHOCH 3CH 2CH(CH 3)CHOCH 3CH(CH 3)CH 2CHOCH 3C(CH 3) 2CHOKetones: CH 3COCH 2CH 2CH 3CH 3CH 2COCH 2CH 3CH 3COCH(CH 3) 2(1 mark each)c)CO+ H 2N ⎯ NHNO 2CN ⎯ NHNO 2+ H 2O(2) Delete 1 mark for each errorNO 2NO 2d) Test Tollens’ reagent / ammoniacal silver nitrate (1)Or Fehling’s solution (1)Conditions Warm (not boil) for Tollens’ reagent (1)Or boil / heat for Fehling’s solution (1)Observation with aldehydes Silver mirror (1)Or red / brown precipitate for Fehling’s solution (1)Observation with ketones No silver mirror (1)Or no red / brown precipitate / solution remains dark blue for Fehling’s solution (1)e) (i) Compare the melting point with that obtained from a data source (1)(ii) Recrystallise (1) to make the compound pure (1),because impurities change/ lower the melting point. (1)
A2 LevelTOPIC 23 ANSWERS & MARK SCHEMES<strong>QUESTIONSHEET</strong> 14THE TRIIODOMETHANE REACTIONa) (i) Yellow (½) precipitate (½)(ii) CH 3CHO + 3I 2+ 3NaOH → CI 3CHO + 3NaI + 3H 2O (1)CI 3CHO + NaOH → CHI 3+ HCOO - Na + (1)(iii) CHI 3is hydrolysed / decomposed by the NaOH (1)(iv) NaClO / ClO - oxidises (1)KI / I - to I 2(1)b) (i) I 2& NaOH oxidises (1)CH 3CHOH to CH 3CO (1)(ii) less reactive/ (1)c) A, E, F, G (1 each)
A2 LevelTOPIC 23 ANSWERS & MARK SCHEMES<strong>QUESTIONSHEET</strong> 15GRIGNARD REAGENTSa) Preparation Magnesium (½) and iodomethane / methyl iodide (½)in dry (½) ether (½) under reflux condenser (½)with iodine (½) as a catalyst (½)Maximum 3 marksEquation CH 3I + Mg → CH 3MgI (1)b) (i) Reagent: methanal (1)Intermediate: CH 3−CH 2OMgl (1)Conditions for second stage: Acidic hydolysis (1)(ii)Reagent: Solid (1) carbon dioxide (1) (Allow ‘Drikold’ or ‘dry ice’ for both marks)OIntermediate: CH 3C (1)OMgIConditions for second stage Acidic hydolysis (1)c) A = propanalB = propanoneC = butan-2-olD = 2-methylpropan-2-ol(Award 1 mark for name or formula in each case )Peak at m/z 29 due to CH 3CH 2+/ C 2H 5+(1)Peak at m/z 30 due to CH(OH) + (1) Not CH 2O +(Deduct 1 mark if +ve charges are missing)
A2 LevelTOPIC 23 ANSWERS & MARK SCHEMES<strong>QUESTIONSHEET</strong> 16a) (i) Product: Potassium ethanoate (1)Type of reaction: Acid-base / neutralisation (1)CARBOXYLIC ACIDS IEquation: CH 3COOH + KOH → CH 3COOK + H 2O (1)(ii) Product: Magnesium ethanoate (1)Type of reaction: Acid-base / neutralisation (1)Equation: CH 3COOH + MgO → CH 3COO) 2Mg + H 2O (1)b) (i) Reagent: Na 2CO 3(aq) / NaHCO 3(aq) (1)Observation: Effervescence / gas evolved which turns limewater milky (1)Equation: 2RCOOH + Na 2CO 3→ 2RCOO − Na + + H 2O + CO 2(1)Or RCOOH + NaHCO 3→ RCOO − Na + + H 2O + CO 2(1)Or similar alternativesc) Reagents Ethanoic acid (1)Butan-1-ol (1)Concentrated sulfuric acid (1)Conditions Heat / boil under reflux (1)Equation CH 3COOH + CH 3CH 2CH 2CH 2OH ¾ CH 3COOCH 2CH 2CH 2CH 3+ H 2O (1)Type of reaction Esterification / addition-elimination / condensation (1)
A2 LevelTOPIC 23 ANSWERS & MARK SCHEMES<strong>QUESTIONSHEET</strong> 17CARBOXYLIC ACIDS IIa) (i) Polarisation of the C=O bond (1)increases polarisation of the O⎯H bond / weakens the O⎯H bond (1)so that there is a greater degree of dissociation / [H + ] is increased (1)(ii) -I effect / electron - withdrawing effect / high electronegativity of Cl atoms (1)further increases polarisation of / weakens the O⎯H bond (1)so that degree of dissociation / [H + ] is increased (1)Also, the anion CCl 3COO - is stabilised (1)by delocalisation of –ve charge (1)Maximum 4 marksb) (i) Reagent PCl 5/ PCl 3/ SOCl 2(1)Equation CH 3CH 2COOH + PCl 5→ CH 3CH 2COCl + POCl 3+ HCl (1)Or 3CH 3CH 2COOH + PCl 3→ 3CH 3CH 2COCl + H 3PO 3(1)Or 3CH 3CH 2COOH + SOCl 2→ CH 3CH 2COCl + SO 2+ HCl (1)(ii) Reagent Conc. NH 3(aq) (1) Accept just ‘NH 3’Equation CH 3CH 2COCl + NH 3→ CH 3CH 2CONH 2+ HCl (1)c) (i) Description Effervescence (1)Gas turns limewater milky (1)at room temperature (1)Maximum 2 marksFormula of product-COO Na+(1) Ionic charges must be shownCOO Na(ii) Description Reaction on heating (1)with conc. H 2SO 4(1)to give a fruity smell (1)Maximum 2 marks-+Formula of productCOOCH 3COOCH 3(1)d) Benzene-1,4-dicarboxylic acid (1)Hydrogen bonding can lead to the formation of “long-chains” of molecules (1)but in benzene-1,2-dicarboxylic acid hydrogen bonding is largely intramolecular (1)
A2 LevelTOPIC 23 ANSWERS & MARK SCHEMES<strong>QUESTIONSHEET</strong> 18a) Name Propanoyl chloride (1)ACYL CHLORIDESTwo advantages Better yield / reaction goes to completion / an equilibrium mixture is not obtained (1)Faster reaction (1)Two disadvantages Acyl chlorides are relatively expensive (1)Fumes of hydrogen chloride / toxic fumes (1)b) (i) Acyl chloride Benzenecarbonyl chloride / benzoyl chloride (1)Reagent Aqueous ammonia (1)(ii) Addition-elimination / condensation (1)(iii) Problem (White) smoke of ammonium chloride (1)Origin Reaction between HCl (formed in the main reaction) and unreacted NH 3(1)Precaution Carry out the experiment in a fume cupboard (1)(Allow ‘limited ammonia’)c) (i) Mix / react ethanoyl chloride and the amine at room temperature (1)Recrystallise the product (1)Determine its melting point (1)Compare this m.p. with that in a data book / textbook (1)Maximum 3 marks(ii) Equation CH 3COCl + C 2H 5NH 2→ CH 3CONHC 2H 5+ HCl (1)Name N-ethylethanamide (1)
A2 LevelTOPIC 23 ANSWERS & MARK SCHEMES<strong>QUESTIONSHEET</strong> 19ESTERSa) O O OH – C H – C CH 3– COCH 2CH 2CH 3OCH(CH 3) 2OCH 2CH 3Propyl methanoate Isopropyl methanoate Ethyl ethanoateor 1-methylethyl methanoateOCH 3CH 2– COCH 3Methyl propanoateAward (1) for each formula and (1) for each name if correctly related to formulaMaximum 6 marksb) (i) Compound II is CH 2OHCH 2OH (1)Compound III is CH 3CH 2CH 2COOH (1)(ii) Compound I Ester (1)Compound II Alcohol / diol (1)Compound III Carboxylic acid (1)(iii) Hydrolysis / saponification (1)(iv) Alkaline hydrolysis goes to completion (1)Acidic hydrolysis is reversible / results in an equilibrium mixture (1)∴ yield is higher from alkaline hydrolysis (1)Maximum 2 marksc) Perfumes (1)Solvents / thinners (1)Food flavourings (1)Plasticisers (1)Maximum 3 marks
A2 LevelTOPIC 23 ANSWERS & MARK SCHEMES<strong>QUESTIONSHEET</strong> 20NITRILESa) n (NaOH) = 0.1 × 16.65/1000 = 1.665 × 10 -3 mol (1)∴ n (H) = 1.665 × 10 -3 mol in 25.0 cm 3= 1.665 × 10 -2 mol in 250 cm 3 (1)∴ M r(H) = 1/1.665 × 10 -2 = 60.06 / 60 (1)b) If formula of H is R-COOH, M rof (R-) is 60.06 – (12 + 32 + 1) = 15.06 (1)∴ R- is CH 3- (1)∴ H is ethanoic acid / CH 3COOH (1)and G must be ethanenitrile / CH 3CN (1)c) (i) CH 3CN + NaOH + H 2O → CH 3COO - Na + + NH 3(1)(ii) CH 3COO - Na + + H 2SO 4→ CH 3COOH + NaHSO 4(1)d) M r(CH 3CN) = (15 + 12 + 14) = 41 and M r(CH 3COOH) = 604.0 g CH 3CN ≡ 4.0 / 41 mol (1)∴ theoretical yield of H is 60(4.0) / 41 = 5.85 g (1)% yield is 4.5(100) / 5.85 = 76.9% / 77% (1)e) Reducing agent LiAlH 4/ H 2/ Zn + dil H 2SO 4(1)Conditions For LiAlH 4, dry ether (1)For H 2, Pt / Ni catalyst (1)For Zn + dil. H 2SO 4, heat / boil (1)Equation CH 3C≡N + 4[H] → CH 3CH 2NH 2(1)OrCH 3C≡N + 2H 2→ CH 3CH 2NH 2(1) only if H 2is quoted as the reducing agentOr CH 3C≡N + 2Zn + 2H 2SO 4→ CH 3CH 2NH 2+ 2ZnSO 4(1)
A2 LevelTOPIC 23 ANSWERS & MARK SCHEMES<strong>QUESTIONSHEET</strong> 21AMIDESa) Heat / boil (1)with bromine (1)and alkali / NaOH(aq) (1)b) (i) Ethanenitrile (1)CH 3CN (1)(ii) Heat / distil (1)with P 4O 10(1)(iii) Elimination (of water) / dehydration (1)c) Smelly compound in Solution A Ethanoic acid (1)Compound in Solution B Sodium ethanoate (1)Gas C Ammonia (1)(Accept formulae in lieu of names)d) (i) Peptide link (1)(ii) Nylon (1)(iii) Protein (1) (Allow ‘polypeptide’)
A2 LevelTOPIC 23 ANSWERS & MARK SCHEMES<strong>QUESTIONSHEET</strong> 22AMINESa) (i) CH 3NH 2+ H 2O ž CH 3NH 3+ OH (1)(ii) The high concentration of OH ions from the NaOH (1)disturbs equilibrium to the left hand side (1)to give molecules of CH 3NH 2, which is a gas (1)Maximum 2 marksb) Name of A Phenylammonium chloride (1)Name of B Phenylamine (1)+ -Equation 1 C 6H 5NH 2+ HCl → C 6H 5NH 3Cl (1)Equation 2 C 6H 5NH 3Cl + NaOH → C 6H 5NH 2+ NaCl (1)-+ -+ -+c) (i) Strength compared with ammonia Stronger (1)Reasons Electron releasing effect / +I effect of C 2H 5group (1)increases electron availability on the N atom (1)so that a lone pair of electrons on N is donated more readily (1)and a proton / H is more strongly accepted (1)Maximum 3 marks(ii) Strength compared with ammonia Weaker (1)Reasons Lone pair of electrons on N is drawn towards the benzene ring (1)so that electron availability on the N is reduced (1)Donation of the lone pair occurs less readily (1)+and a proton / H is less strongly attracted (1)Maximum 3 marksd) (i) Substitution / replacement of H on the N atom (1)by an acyl group / RCO (1)(ii) Ethanoyl chloride / ethanoic anhydride / benzenecarbonyl chloride (1)(iii) N-substituted amide / substituted amide / amide (1)(iv) Mark consequentially from (ii), e.g.CH 3COCl + C 6H 5NH 2→ CH 3CONHC 6H 5+ HCl (1)Accept C 6H 5NHCOCH 3
A2 LevelTOPIC 23 ANSWERS & MARK SCHEMES<strong>QUESTIONSHEET</strong> 23DIAZOTISATIONa) (i) Treatment of a primary aromatic amine with nitrous acid to form a diazonium salt (1)(ii) Compounds needed for diazotisation Sodium nitrite (1) and hydrochloric acid (1)Name of product Benzenediazonium chloride (1)Equation C 6H 5NH 2+ NaNO 2+ 2HCl → C 6H 5N≡N Cl + NaCl + 2H 2O (1)(iii) Optimum temperature 0-10 0 C (1)Problem if the temperature were too high Hydrolysis of benzenediazonium chloride (1)to give phenol (1)or decomposition of benzenediazonium chloride (1)to give chlorobenzene (1)Problem if the temperature were too low Slow / low rate of reaction (1)b) (i) Conditions Cold / ∼ 5 0 C (1)In alkaline solution / NaOH(aq) (1)+ -ObservationEquation(ii) Coupling (1)Red (½) precipitate (½)OH++N≡N Cl(1) (1)-N=NOH+ HCl (1)c) A would give a red precipitate (1)but B would not (1)
A2 LevelTOPIC 23 ANSWERS & MARK SCHEMES<strong>QUESTIONSHEET</strong> 24AMINO ACIDSa) RH 2N –C – COOH (1)Hb) Glycine forms zwitterions (1)NH 3– CH 2– COO (1)+ -+ +Attraction between zwitterions is stronger (1)than hydrogen bonding between molecules of glycollic acid (1)c) (i) Equation (low pH) H 2N – R – COOH + H ž H 3N – R – COOH (1)Equation (high pH) H 2N – R – COOH + OH ž H 2N – R – COO + H 2O (1)(ii) Amino acids exists as zwitterions (1)which are attracted equally strongly to both electrodes (1)- -d) (i) CH 3CH 2OH CH 3CH 2OHH 2N – CH – COOH + H 2N – CH – COOH → H 2N – CH – CONH – CH – COOH (1)CH 2OH CH 3CH 2OH CH 3H 2N – CH – COOH + H 2N – CH – COOH → H 2N – CH – CONH – CH – COOH (1)(ii) They are amino acids because they both contain –NH 2and –COOH groups (1)but they are not α-amino acids because these groups are attached to different carbon atoms (1)
A2 LevelTOPIC 23 ANSWERS & MARK SCHEMES<strong>QUESTIONSHEET</strong> 25PROTEINSa) (i) Name Peptide link (1)O HFormula – C – N – (1)(ii) Condensation polymerisation (1)(iii) Amino acid residues can be in different sequences (1)(iv) Protein molecules contain > 30 – 40 amino acid residues / polypeptides contain < 30 – 40 (1)Protein molecules are always hydrated / polypeptides are not hydrated (1)Maximum 1 markb) Hydrogen bonding holds a protein chain in a coil or spiral / α-helix (1)Water molecules are held to a protein chain by hydrogen bonding (1)c) (i) Hydrolysis (1)Heat / boil under reflux (1)with concentrated aqueous acid / hydrochloric acid (1)(ii) Chromatography (1)d) They undergo hydrolysis (1)due to the catalytic action of digestive enzymes (1)
A2 LevelTOPIC 23 ANSWERS & MARK SCHEMES<strong>QUESTIONSHEET</strong> 26TEST QUESTION 1CARBONYL COMPOUNDSa) C 19H 28O 2b) (i) Red/yellow/orange precipitate (1)(ii) Orange to green (1)(iii) Orange/brown to colourless or decolourised (1)c)CH 3OHCH 3HO1 mark for the HO group and 1 mark for the rest of the molecule.d)CH 3OCH 3O1 mark for the C = O group and 1 mark for the rest of the molecule.e)OHO(1)f) (i) B (1)(ii) A (1)
A2 LevelTOPIC 23 ANSWERS & MARK SCHEMES<strong>QUESTIONSHEET</strong> 27TEST QUESTION 1IPHENOLSa) Ketone (1)b) C 14H 8O 4(1)c) C 7H 4O 2(1)d) (i)OO − Na +OO − Na +1 mark for both Na + ions and 1 mark for the rest of the compound(ii)The compound is ionic (1) and attracts water molecules (1), but quinizarin is non-polar and does not attract watermolecules (1)e)Br Br BrH O CHBrCH 21 mark for the addition of Br and 1 mark for the substitution of Br