Solutions
Solutions
Solutions
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
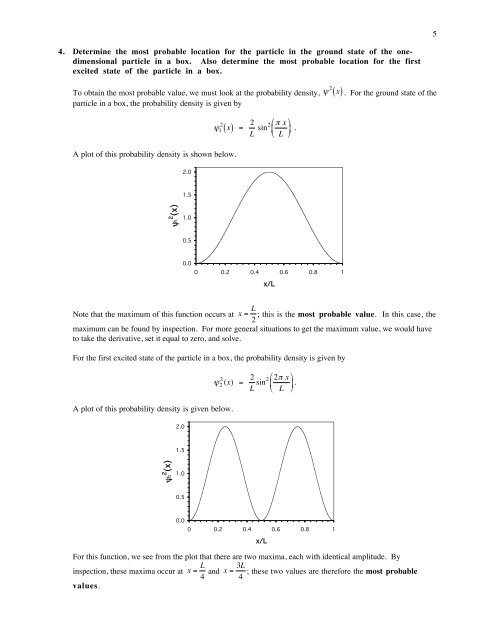
54. Determine the most probable location for the particle in the ground state of the onedimensionalparticle in a box. Also determine the most probable location for the firstexcited state of the particle in a box.To obtain the most probable value, we must look at the probability density,particle in a box, the probability density is given by€( ) = 2 π xL sin2 ⎜⎜ψ 1 2 xA plot of this probability density is shown below.2.0⎛⎛ ⎞⎞⎟⎟ € .⎝⎝ L ⎠⎠ψ 2 ( x) . For the ground state of the1.5ψ12 (x)1.00.50.00 0.2 0.4 0.6 0.8 1x/LNote that the maximum of this function occurs at x = L ; this is the most probable value. In this case, the2maximum can be found by inspection. For more general situations to get the maximum value, we would haveto take the derivative, set it equal to zero, and solve.€For the first excited state of the particle in a box, the probability density is given byA plot of this probability density is given below.€2.0ψ 2 2 (x) = 2 ⎛⎛ 2π x ⎞⎞L sin2 ⎜⎜ ⎟⎟ .⎝⎝ L ⎠⎠1.5ψ22 (x)1.00.50.00 0.2 0.4 0.6 0.8 1x/LFor this function, we see from the plot that there are two maxima, each with identical amplitude. Byinspection, these maxima occur at x = L 4 and x = 3L ; these two values are therefore the most probable4values.€€