Multicomponent Reactions - The Stoltz Group
Multicomponent Reactions - The Stoltz Group
Multicomponent Reactions - The Stoltz Group
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
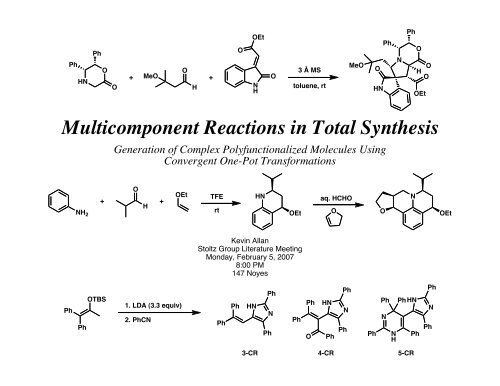
PhHNPhOOEtOO+ MeO+OO HNH3 Å MStoluene, rtMeOOHNPhNPhOOHOOEt<strong>Multicomponent</strong> <strong>Reactions</strong> in Total SynthesisGeneration of Complex Polyfunctionalized Molecules UsingConvergent One-Pot TransformationsNH 2O+ +HOEtTFErtHNOEtaq. HCHOOONOEtKevin Allan<strong>Stoltz</strong> <strong>Group</strong> Literature MeetingMonday, February 5, 20078:00 PM147 NoyesPhPhOTBS1. LDA (3.3 equiv)2. PhCNPhPhHNPhNPhPhPhOHNPhPhNPhPhPhNNHPhHNPhPhNPh3-CR 4-CR 5-CR
<strong>Multicomponent</strong> <strong>Reactions</strong>A New Approach to Total Synthesis· Linear total syntheses require significant amounts of time and money to advance starting materials to complex targets.BCADA1 molA B1 mol 1 mol50%50% 50%BC1 mol 0.5 mol 0.25 mol 0.125 molBACD· MCRs minimize cost in the form of time and material by generating complex targets in a single convergent step.AA + B + C + D50%BDC1 mol1 mol1 mol1 mol0.5 mol· MCRs typically run at mild temperatures and do not require many reagents in excess of the participating substrates.This has made them ideal for combinatorial library synthesis as well as industrial pharmaceutical synthesis.(1)(2)···(n)A 1A 2A n B 1B 2B n C 1C 2C n D 1D 2D n· + · + ····+······A 1B 1 D 1C 1···B nA nC nD nm component reaction with n types of each component → n m productsn = 5 (20 SM cmpds) → 625 productsn = 10 (40 SM cmpds) → 10,000 productsn = 50 (200 SM cmpds) → 6.25 mill products
Mannich ReactionThree-Component Synthesis of β-Aminocarbonyls· In 1903, B. Tollens and C. M. van Marle observed the formation of a tertiary amine from the treatment of anaqueous solution of acetophenone and formaldehyde with ammonium chloride.O+HOHNH 4 ClH 2 OTollens, B.; van Marle, C. M. Ber. 1903, 36, 1347-1351.· In 1917, Carl Mannich (Berlin, Germany) reported the formation of a number of β-aminoketones under the sameconditions.OOO+ +H HHNH 2 O100 °CON3NOO+ +H HNH 4 ClH 2 O100 °Cseveral productsOOO+ +H HNH 4 ClH 2 O100 °CN+ several side productsMannich, C. Archiv. Pharm. 1917, 255, 261-276.Mannich, C. J. Chem. Soc. Abstr. 1917, 112, 634-635.· <strong>The</strong> Mannich reaction is a Type I MCR that proceeds under both acidic and basic conditions, though acidic conditionsare more common. In 1969, Stephen Benkovic (Penn State) performed kinetics experiments over a broad pH rangethat supported the formation of a pH-dependent steady state iminium intermediate under acidic conditions. Underbasic conditions, a carbinolamine intermediate presides.HNNHHNCO 2 EtLewis or Bronsted acidPolar solventBaseApolar solventHNNHNNHNArHNNNCO 2 EtHOHNArBenkovic, S. J. J. Am. Chem. Soc. 1969, 91, 1860-1861.
Mannich ReactionSelect Total SynthesesRobinson's Synthesis of Tropinone:NOHOOAtropineNOOMeOOCocaineO O OHOOH+Me NH 2+H HOOH 2 Ort, 50 hNHOHOOOO- CO 2NORobinson, R. J. Chem. Soc. 1917, 762-768.Omura's Synthesis of (+)-Madindoline A and B:HOTMSONH+MeOHCO 2 Me37% aq. HCHOSc(OTf) 3 (10 mol%)H 2 O, rt, 24 h(11% yield)HONOTMSOHMeCO 2 Men-BuTAS-FDMF, rt, 20 min(77% yield)HONOn-BuOHMeO+OHONOHMeOn-BuTMSn-BuTAS-F =(Me 2 NH) 3 S Me 3 SiF 2(+)-Madindoline A(+)-Madindoline BOmura, S. Tetrahedron Lett. 2006, 47, 6761-6764.
Petasis (Boronic Acid Mannich) ReactionThree-Component Synthesis of Allyl, Propargyl, and Benzylamines· In 1993, Nicos A. Petasis (USC) described the preparation of tertiary allylic amines from paraformaldehyde, secondaryamines, and vinyl boronic acids under acid-free conditions. Yields ranged from 75-96%.NHOHN+BOH(CH 2 O) ndioxane90 °C, 30 minOOH+BNH n-C 5 H 11 OHPetasis, N. A. Tetrahedron Lett. 1993, 34, 583-586.ON n-C 5 H 11· <strong>The</strong> Petasis reaction is a Type II reaction, though the precise mechanism is still disputed:· Petasis noted that in the absence of acid, it was unlikely that significant amounts of the iminium salt would form.Also, boronic acids did not readily add to preformed iminium salts, though they did react with diamines. Based onthese observations, Petasis proposed the formation of an intermediate ammonium borate complex followed byinternal delivery of the vinyl nucleophile.(CH 2 O) ndioxane90 °C, 30 minHOHOOHBOHBRR++NNClNSLOWFASTNNRRHOOHBR 2 NH+R 3R 1HOHR 1HOOH R 2 HB O N R 3H HR 2R 1 NR 3· However, the vast majority of cases in the literature employ an aldehyde substrate containing a directing group nearthe carbonyl, indicating a necessity for formation of a two-center ionic complex. In these cases, a protic solventis typically used (MeOH, EtOH, TFE).ROOHHNOHHOOOHROOHOROOHHR 2 +NHR 3HOOHBRRR 2 R 3NOHR 1B OHOHRR 2 R 3NROHBryce, M. R.; Hansen, T. K. Tetrahedron Lett. 2000, 41, 1303-1305.Schreiber, S. L. Angew. Chem. Int. Ed. 2006, 45, 3635-3638.Petasis, N. A. Tetrahedron Lett. 2001, 42, 539-542.McReynolds, M. D.; Hanson, P. R. Chemtracts 2001, 14, 796-801.
Petasis (Boronic Acid Mannich) ReactionPyne's Total Synthesis of Uniflorine AHOHOOHOL-xyloseOH+H 2 NHOBOHPhEtOHrt, 16 h(73% yield)HOHHOPhHNHOOHsingle diastereomer1. Boc 2 O, Et 3 N2. TrCl, pyr3. Grubbs 1 st gen.CH 2 Cl 2 , reflux(30% yield, 3 steps)HOHOHOTrONHBoc1. K 2 OsO 4·2H 2 O, NMOacetone / H 2 O, rt2. NaH, BnBr(67% yield)BnOBnOBnOTrOHOBnOBnNBocTFAanisole, CH 2 Cl 20 °CBnOBnOBnOHOBnBnOHOBnBnOOBn +OBnHNNBnOHO37% yield 54% yieldPdCl 2 , H 2MeOH, rt(63% yield)HOHOHOHNOHUniflorine AOHconverted to peracetatefor crystallographyPh 3 P, CBr 4Et 3 N, CH 2 Cl 2 , 0 °C(54% yield)Pyne, S. G. J. Org. Chem. 2004, 69, 3139-3143.
Biginelli ReactionThree-Component Synthesis of Substituted Dihydropyrimidinones· In 1891, Pietro Biginelli (Florence, Italy) discovered a three-component condensation of aldehydes, ureas, andβ-ketoesters under strongly acidic conditions that produced dihydropyrimidinones.PhHPhO+EtO 2 CO+H 2 NNH 2Ocat. HClEtOH, refluxEtO 2 C*NHNHOBiginelli, P. Ber. 1891, 24, 2962-2967,Biginelli, P. Gazz. Chim. Ital. 1893, 23, 360-416.· In 1973, Sweet et al. proposed a Type II mechanism in which the first, rate-limiting step involved aldol condensation ofthe β-ketoester and aldehyde substrates, followed by S N 1 loss of H 2 O to form a β-carbocation. Quenching with ureaand condensation with the ketone then closed the ring.EtO 2 CO+HPhOH +- H 2 OEtO 2 CPhOH 2 NNH 2O- H +EtO 2 CPhONHONH 2- H 2 OEtO 2 CPhNHNHOSweet, F.; Fissekis, J. D. J. Am. Chem. Soc. 1973, 95, 8741-8749.· In 1997, Kappe et al. proposed at alternate mechanism in which the urea and aldehyde substrates first condensedto form an N-acyliminium intermediate that was then quenched by addition of the β-ketoester.HPhO+H 2 NNH 2OH +- H 2 OPhNHNH 2OEtO 2 COEtO 2 CPhNHONH 2O- H 2 OEtO 2 CPhNHNHOKappe, C. O. J. Org. Chem. 1997, 62, 7201-7204.Kappe, C. O. Acc. Chem. Res. 2000, 32 879-889.
Biginelli ReactionApplication to the Total Synthesis of the Batzella AlkaloidsNH 2 OH HNH 2 OH HNNHONNNHONH 2 N6 MeHNO ONH 2Batzelladine ANHNH(CH 2 ) 8 MeH 2 NHNNH 2OMeOBatzelladine B6NHNH(CH 2 ) 8 MeH 2 NHNOONHH 2 NHNOOHNHNH 2NNH(CH 2 ) 6 MeNH 2MeNHNH(CH 2 ) 8 MeDehydrobatzelladine CBatzelladine DMeHNHNNHHOOMeHNHNNHH(CH 2 ) 8 MeH 2 NH 2 NNO14OOHONHNNHHOBatzelladine FPtilomycalin AH 2 NH 2 NOHNO14OOHONHNNHHOH 2 NH 2 NOHNOHOOONNNHHOCrambescidin 800CrambidineIsolations:Batzelladines A-E: Patil, A. D. J. Org. Chem. 1995, 60, 1182-1188.Batzelladines F-I: Patil, A. D. J. Org. Chem. 1997, 62, 1814-1819.Ptilomycalin A: Kashman, Y. J. Am. Chem. Soc. 1989, 111, 8925-8926.Crambescidin 800: Minale, L. Tetrahedron 1995, 51, 3675-3682.Crambidine: Kashman, Y. J. Am. Chem. Soc. 1992, 114, 8472-8479.
Biginelli Reaction2-Component 3-Center: Overman's Synthesis of (—)-Batzelladine DO11 steps(47% yield)MeOMeOH 2 NNHOHn-C 9 H 19AcOHH 2 OH 2 NNNHOHn-C 9 H 19NH·HClOOO(CH 2 ) 4 NHCbzCbzHN(CH 2 ) 4 OOHNOHn-C 9 H 191. MsCl, Et 3 NCbzHN(CH 2 ) 4 OOHNHONH·HOAcMeNHNH·HOAc2. Et 3 N, CHCl 3refluxMeNHNH(CH 2 ) 8 Me(55% yield, 2 steps)(60% yield) ClH 2 , Rh/Al 2 O 3H 2 N(CH 2 ) 4 OOHNHHCl·HNNNNH 2H 2 NHNOOHNHHCO 2 H, MeOH/H 2 O(68% yield)MeNHTFANH(CH 2 ) 8 Mei-Pr 2 NEt, DMFNH 2(75% yield) TFAMeNHTFANH(CH 2 ) 8 Me1:1.2 d.r.(desired is minor)(—)-Batzelladine DOverman, L. E. J. Org. Chem. 1999, 64, 1512-1519.Overman, L. E. Org. Lett. 1999, 1, 2169-2172.
Biginelli ReactionModified Biginelli: Kishi's Total Synthesis of (±)-SaxitoxinOBnHNCO 2 MeOO+S·NSi 4PhHRHNSNCO 2 MeOOHOOBnSNNCO 2 MeOO100 °C(75% yield)OBnOBnOBnSHNNCO 2 MeOO1. NH 2 NH 2·H 2 O, MeOH2. NOCl, CH 2 Cl 2 , - 50 °C3. PhH, 90 °C4. NH 3 , PhH(75% yield, 4 steps)SHNNHN NH 2OOOHS SHBF 3·OEt 2(63% yield)SHNNHN NH 2OSSTFA / AcOH50 °C(50% yield)OBnOHH 2 NOSHNNHNNHSO1. Et 3 O·BF 4 , NaHCO 32. EtCO 2 NH 4 (33%, 2 steps)3. BCl 3 , 0 °C (75%)4. NBS, MeCN / H 2 O (30%)HNHNNHNNOHNH 2O·N SO 2Clhot H 2 Owork-upOHNHNNHNNOHNH 2SOH(50% yield)(±)-SaxitoxinOHBusby, G. W., III. Ph.D. Dissertation, Harvard University, 1974.Kishi, K. Y. J. Am. Chem. Soc. 1977, 99, 2818-2819.
Passerini ReactionSemple's Total Synthesis of Eurystatin ABocHNBocHNONHCbzOBnMeFmocHNN1. Et 2 NH, CH 2 Cl 2CH 2 Cl 2H(75% yield)OH + H +OBnO O OFmocHNCN0 °C → rt2. H 2 , Pd/COO3-5 daysNHCbz MeOHBocHN(80% yield)(95% yield)HO OO Me O1. HCl, EtOAc, 0 °COH DPPA, NaHCO 3(quantitative)NNHN HNHHDMF, 0 °COH OOO2. EDC, AcOH, DMF(0.005 M)5 daysNHBocHNOHNH 2 (75-85% yield)O(93% yield)MeOHOOOOOOHNNHHNNHOpyr·SO 3Et 3 N, DMSO5 °C(75% yield)OOHN HNNHNHEurystatin AO· 9-10 steps fromcommercial amino acids· 20-26% overall yieldSemple, J. E. Tetrahedron Lett. 2001, 42, 6271-6274.
Passerini Reaction2-Component 3-Center: Falck's Four-Step Synthesis of (±)-HydrastineOOOOMeOHONHOHNCO 2 HOOHONHOOMeOOMeOHOONoscapine(+)-Coryximine(+)-EgenineOOOBrLiCH 2 NCTHF- 78 °C → rt(88% yield)OONCMeOMeOOCH 2 Cl 2 , rt3 h(71% yield)HOHOOOONHOOOMeOOMeNHO1. POCl 3 , MeCNH HNaBH 3 CN2. PtO O2 , H 2 HCHOOHONHapprox. 1:1 mixtureof naturally interconvertingisomersOMeOOMe(58% yield,3 steps)OMeOOMe(±)-HydrastineFalck, J. R. Tetrahedron Lett. 1981, 22, 619-620.
Ugi ReactionFour-Component Synthesis of α-Acylamidoamides· In 1959, Ivar Ugi (Ludwig Maximilians Universität, Munich) discovered the four-component coupling of isocyanides,carboxylic acids, amines, and aldehydes/ketones to form α-acylamidoamides.R 1 OR 2O RO1 R 2solvent+ R 3 NH 2+ + R 5 NCR 4 R 4 NOHR 3 ONHR 5"Had Passerini been conversant with present day views on reaction mechanisms while studying the reactionwhich now bears his name, he would probably have added ammonia or the primary amines to his three startingmaterials and thereby discovered the α-amino alkylation of isonitriles and acids."Ugi, I. Angew. Chem. 1959, 71, 386.Ugi, I. Angew. Chem. 1960, 72, 267-268.· <strong>The</strong> reaction is a Type II MCR and the mechanism mirrors that of the Passerini reaction, only preceded by thecondensation of the carbonyl and amine species.R 1 O+ R 3 NHR 2 2- H 2 OR 1 NR 3 R 2 R 5 NCONHR 3 NHR 3R 4 OR 1 R 1 OR 2 NR 5 R 2NR 5OOacylR 1 R 2R 4 migration R 4 NR 3 ONHR 5· <strong>The</strong> Ugi reaction has been applied to the synthesis of a broad range of product structures by substituting theamine and carboxylic acid components.R OR SR SeNR 3 NHR 5NR 3 NHR 5NR 3 NHR 5R 1 R 2 R 1 R 2 R 1 R 2R NR 6NR 3 NHR 5R 1 R 2R 4 OOH NR 6NNHR 5R 1 R 2R = H, R 4 OR 6 R 7N OR O OR 4 NNHR 5NR 3 NO R 1 R 2R 1 R 2 R 5R 6R NNR 3 R 1 R 2NNNR 5R 3 R1 NR 2XNNR 5HUgi, I.; Dömling, A. Endeavour 1994, 18, 115-122.X = O, S, NR 6
MeOMeOMsOOMeOOONNH 2OTBDPSNBocMeOMeO+HO 2 COHNHBocCN1. NaBH 4 , THF / H 2 OUgi ReactionFukuyama's Route Toward (—)-LemonomycinOOOPhMeOOAcTFE25 → 50 °CNBocTMSMeOMeOMeOMeOMsOBF 3·OEt 2NHNOONHBocOTBDPSOMeOOOPh1. CSA, quinoline(73% yield, 2 steps)2. KOt-BuNBocTMSMeOMsONOOTBDPS2. TBAF, Ac 2 O, pyr3. Grubbs 2nd gen.TMSMeOMsONOOAc(95% yield)MeOMsONOOAc(35% yield, 4 steps)MeOMeOMeOHOOMeMeOMsONOOAcNBoc1. TMSOTf2. CbzCl, DMAP3. K 2 CO 3 , MeOH4. TIPSOTf, 2,6-lutidine(55% yield, 4 steps)MeOMsONOOTIPSNCbzDMDOMeOHMeOMsONOOTIPSNCbz1. NaBH 3 CN, TFA2. KOTMS, MeCN, 0 °Cthen BnBr, 50 °C(53% yield, 3 steps)MeOMeOBnOHOHNOOTIPSNCbz1. DMP, CH 2 Cl 22. TFA, CH 2 Cl 2Fukuyama, T. Chem. Commun. 2005, 394-396.(76% yield, 2 steps)MeOMeOBnOHOHN9:1 d.r.OOTIPSNCbzOMeOO(—)-LemonomycinHOOHHNHNOHOOHON
Ugi ReactionBeyond Four-Component <strong>Reactions</strong>· Five-component reaction:OMeOH + CO 2+ n-Bu NH 2 +H+CH 2 NMeOONHNn-BuOHaslinger, E. Monatsh. Chem. 1978, 109, 749-750.· Six-component (seven-center) reaction (Mannich-Ugi):HOHHH+ + N + MeOH +OH 2 NHOOH+MeNCNHNOMeONHMeOUgi, I.; Dömling, A.; Hörl, W. Endeavour 1994, 18, 115-122.· Seven-component reaction (Asinger-Ugi):NaSH+BrOHH+ NH 3 + + CO 2+ MeOH +ONCNHSNOOOMeUgi, I.; Dömling, A.; Hörl, W. Endeavour 1994, 18, 115-122.
Povarov ReactionThree-Component Synthesis of Substituted Tetrahydroquinolines· In 1965, L. S. Povarov discovered a Lewis acid-catalyzed cycloaddition between N-aryl imines and vinyl ethers.OR 2ROR 2 R Lewis+3HN R 1 acidR 4RNHR 3 R 4R 1Povarov, L. S.; Mikhailov, B. M. Izv. Akad. Nauk SSSR, Otd. Khim. Nauk 1963, 955.Povarov, L. S.; Mikhailov, B. M. Izv. Akad. Nauk SSSR, Otd. Khim. Nauk 1963, 2039.Povarov, L. S. Russ. Chem. Rev. 1965, 34, 639.Povarov, L. S. Doctoral <strong>The</strong>sis, Institute of General and Inorganic Chemistry, Academy of Sciences of the USSR, Moscow, 1966.· <strong>The</strong> mechanism is disputed, though classified as Type II due to the irreversibility of rearomatization in the final step.· Though casually referred to as an aza-Diels-Alder cycloaddition, the Povarov is most often proposed as anon-concerted, two-step ionic mechanism. This is supported by trapping experiments (Type I).OOOO 2 NNH 2+HOCO 2 EtO 2 NN CO 2 EtSc(OTf) 3O 2 NHN CO 2 EtHSc(OTf) 3MeOHCO 2 EtHO 2 N NHMeO OLavilla, R. Angew. Chem. Int. Ed. 2005, 44, 6521--6525.· However, a step-wise mechanism does not explain the observation that only one stereoisomeric product isgenerated in reactions with dihydrofuran and dihydropyran.OOOORN RHsingle diastereomerRN RLewis acidLewis acidLucchini, V. J. Chem. Soc. Perkin Trans. I 1992, 259-266.RNHRsingle diastereomer
Povarov ReactionSubstrate ScopeOEtNH 2+HOO+OEtNHOH 2 NXNH 2X = H, Me, CO 2 HNH 2NH 2O+ H++HOO+ H+O+ H+SRR = Et, n-BuO+ ·HNOHNNH 2 NHOMePhXSRNHONHHNNHPhPhPhMeOPhNNHR 1R 1R 2R 2· <strong>The</strong> carbonyl component is typicallylimited to aryl substituents due totautomerization of aliphatic imines tothe corresponding enamines.· Original conditions used p-TsOH asthe catalyst and heated to 100-200 °C.· More recently, transition metal andlanthanide triflates have been shownto catalyze the Povarov reactionbetween RT and 50 °C.ONH 2+ +OMeONHPovarov, L. S. Russ. Chem. Rev. 1967, 36, 656-670.Batey, R. A. Tetrahedron Lett. 2003, 44, 7569-7573.Lavilla, R. J. Comb. Chem. 2005, 7, 33-41.Legros, J. Synlett 2006, 1899-1902.
Povarov ReactionTotal Syntheses of (±)-Martinellic Acid and (±)-MartinellineNHONHNHONHHNNHHNMartinellic AcidNHH 2 NNHHNOONHNNHHNNHHNMartinelline· Root extracts of the Martinella iquitosensis vine, found in Amazonian lowland rainforests.· Used by indigenous peoples to treat eye ailments, including inflammation and conjunctivitis.· Display modest antibiotic activity and micromolar binding of G-protein coupled receptors.Cook, K. J. Ethnopharmacol. 1984, 11, 337-343.Witherup, K. M.; Varga, S. L. J. Am. Chem. Soc. 1995, 117, 6682-6685.
Stevenson's Approach to the Tricyclic Core:Povarov ReactionTotal Syntheses of (±)-Martinellic Acid and (±)-MartinellineOCbzNOCbzNOCbzNMeO+HMeO+MeONH 2OPhNHPhNHPhConditions:Batey's Approach (Modified Povarov):A. InCl 3 (12 mol%), MeCN, 25 °C, 12 hB. Y(OTf) 3 (1 mol%), MeCN, 25 °C, 2 hC. LiBF 4 (100 mol%), MeCN, 25 °C, 12 h→ 40% yield, 1.1:1 d.r.→ 47% yield, 1:1 d.r.→ 43% yield, 1:1 d.r.Stevenson, P. J. Tetrahedron 2006, 62, 3977-3984,MeOOCbzN+NH 21 equiv 2 equivMeOOCbzNNHExoNHCbz6 stepsMartinellineMeOONNHCbz3MeOOCbzNNH+EndoNHCbzConditions:Dy(OTf) 3 : 92% yield15:85 exo:endoCSA: 74% yield89:11 exo:endoBatey, R. A. Org. Lett. 2002, 4, 2913-2916.
Povarov Reaction2-Component 3-Center: Batey's Formal Total Synthesis of CamptothecinMeOMeOO+MeONOMeDMF, 80 °C, 3 hthenONCNH 2MeOMeOHNOCN1. K 2 CO 3 , LiBrBu 4 NBr, tol:H 2 O (100:1)100 °C, 1 h2. AcOH / HCl / H 2 O60 °C, 1 hOHNOCNDMF, 100 °C, 3 h(86% yield)(29% yield)OHNONH 2Dy(OTf) 3 (10 mol%)NNO[4+2]NOCNMeCN, 50 °C, 16 hCNNCN71% yieldEckertNNOOOHOCamptothecinBatey, R. A. Org. Lett. 2004, 6, 4913-4916.Eckert, H. Angew. Chem. Int. Ed. Engl. 1981, 20, 208-210.
Knoevenagel Condensation / Hetero-Diels-Alder CycloadditionTietze's Total Synthesis of HirsutineONR+ONCbzOHEDDANRO∗NCbzOOPMBNRO∗NCbz∗OOPMBOOOOOOR = H or BocH 2 O- CO 2, acid-ONR∗NCbz∗OOPMBH 2 , Pd/CK 2 CO 3 , MeOHNRO∗N∗1. (remove Boc)2.OH OMe3. CH 2 N 2NHOO3NOMe15 (3R,15R) : Hirsutine(3S,15R) : DihydrocorynantheineOMeOMeR = Boc, 67% yield= H, 62% yield(converted to Boc beforemethanolysis)Tietze, L. F. Synthesis 1998, 1185-1194.Tietze, L. F. Angew. Chem. Int. Ed. 1999, 38, 2045-2047.
Knoevenagel Condensation / Hetero-Diels-Alder CycloadditionHirsutine: Origin of Stereoselectivity· Either enantiomer—(3R) or (3S)—is available by condensation of piperidyl nitrogen with (—)-camphanic acid to formseparable diastereomeric amides. Enantiomerically pure (3R) substrate advanced to hirsutine.· During the cycloaddition, N-Boc causes Meldrum's acid moiety to swing away from indole, while N-H causes it toswing toward indole in order to participate in H-bonding and partial donation of the nitrogen lone pair into the enoateπ-system.· <strong>The</strong> enol approaches the (E)-1-oxa-1,3-butadiene over the H at C(3) instead of the alkyl chain.NBoc3 NCbz3NHH15 15NCbzHOOOOOOOOβ approachto(E)-enoateOPMBβ approachto(E)-enoateNH3N15OHOPMBPMBOH15 15OOONH3N15OOMeOOOOOOMeOMeHirsutineTietze, L. F. Synthesis 1998, 1185-1194.Tietze, L. F. Angew. Chem. Int. Ed. 1999, 38, 2045-2047.OMe15-epi-Hirsutine
Dipolar CycloadditionsMethods for Dipole Construction<strong>Multicomponent</strong> dipolar cycloadditions typically involve two-component formation of the 1,3-dipole followed by reactionwith a suitable dipolarophile.R 1 O+R 2 HN-OHYb(OTf) 3R 2R 1 NO+R 3CO 2 RCO 2 RR2 N O R 3R 1RO 2 CCO 2 RKerr, M. A.Org. Lett. 2004, 6, 139-141.R 1R 1N+BrOR 2Al 2 O 3µwavesOR 2N+CO 2 RR 3OR 1 Boruah, R. C.OOrg. Lett. 2003, 5, 435-438.NMüller, T. J. J.ORHelv. Chim. Acta 2005, 88, 1798-1812.R 3R 2ArNR 1O ORO2[CuOTf] 2·C 6 H 6+ HArOR 2+NN 2R 1CO 2 RCO 2 RCO 2 R 2Ar CON2 RScheidt, K. A.Org. Lett. 2003, 5, 3487-3490.R 1CO 2 RR 1 O+ROON 2OORCO 2 RRO 2 C CO 2 RRh 2 (OAc) 4 ArArO CO 2 RONair, V.+J. Org. Chem. 2004, 69, 1413-1414.R 1 O 2 NR 1 NO 2R 1NC+CO 2 RCO 2 RN R1HN R1Δ NC CNCNRO 2 CRO 2 CNair, V.+Org. Lett. 2004, 6, 767-769.CNArRO 2 CRO 2 C Ar
Dipolar CycloadditionsWilliams' Total Synthesis of (—)-Spirotryprostatin BPhPhOPhPhHNPhOOHOEtO 2 COMe3 Å MS, tolueneMeONOHN+OCO 2 EtMeOOHNPhNOHOCO 2 EtNHOβ-exo transition state82% yieldsingle diastereomer8 stepsONNOOHN(—)-Spirotryprostatin B· 16% overall yield· (+)-spirotryprostatin available from enantiomerof oxazinone substrate.· Schreiber later used this reaction to construct a library of >3000structural variants using split-pool synthesis on microbeads.i-Pr i-PrSi LinkerOHNPhNPhOHOCO 2 NR 1 R 2Williams, R. M. J. Am. Chem. Soc. 2000, 122, 5666-5667.Williams, R. M. Tetrahedron 2002, 58, 6311-6322.Schreiber, S. L. J. Am. Chem. Soc. 2004, 126, 16077-16086.R 3
Dipolar CycloadditionsKerr's Total Synthesis of (+)-Nakadoumarin ABrOBnTBDPSOO+HONHOHYb(OTf) 3(15 mol%)4 Å MStol, 100 °CPMBHNOfuranMeO 2 CCO 2 MesingleenantiomerTBDPSOPMBON OBnOCO 2 MeBr CO 2MeMeO87% yield(single diastereomer)1. DIBAL, CHCl 2 , -78 °C2. O OMeOPMeO3. Pd(PPh 3 ) 4 , Ag 2 SO 4Et 3 N, DMF, refluxOMe, KOt-Bu(66% yield, 3 steps)PMBN OOOTBDPSBnOCO 2 MeCO 2 Me1. DDQ2. OCl3. SmI 24. MsCl4. KOt-BuOBnO NOOTBDPSOBnOBnCO 2 MeCO 2 MeOBnOBn1. NiCl 2·6H 2 O, NaBH 42. LiAlH 43. MsCl4.NH 2OTBDPSKerr, M. A. Org. Lett. 2005, 7, 953-955.Kerr, M. A. J. Am. Chem. Soc. 2007, ASAP.O NOOTBDPSNOTBDPS9 stepsN HHONH(+)-Nakadoumarin A· 22 steps· Unnatural enantiomer· Absolute stereochem.of cyclopropanedetermines absolutestereochem. of product
Multiple Anion CaptureDianion Relay for Structural ElaborationMultiple anion capture is a process by which a resonance-stabilized dianion attacks a relais ("relay", electrophile) speciesto form a secondary dianion, which is then quenched by a second electrophile.· Relais species =· Dianions =PhPhO1. KHRNNORPh2. n-BuLi PhKROLiCNO"Y-shaped" dianionNHPhRON ·PhO· If the second electrophile contains one electrophilic site, then two equivalents are necessary and the product is a linearhomologation of the substrate.OPhRPhKN ·OLiSOPhPhRR·RRMe 2 SiClRRMe 2 SiOPhPhOSiMe 2 RPhPh86% (R = Me)84% (R = t-Bu)· If the second electrophile contains two electrophilic sites, then only one equivalent is necessary and the product is a ring.NArArOPhPhPh PhOSiOPhPhO OPhPh 2 SiCl 2Ph (COCl) 2PhPhK LiPhOPhOOOPhPh76% yieldLanger, P. Eur. J. Org. Chem. 2001, 1511-1517.Langer, P. Chem. Eur. J. 2001, 7, 3858-3866.Padwa, A. J. Am. Chem. Soc. 1988, 110, 1617-1618.Ketchum, R. J. Org. Chem. 1985, 50, 3431-3434.Trimitsis, G. B. J. Org. Chem. 1983, 48, 2957-2962.36% yield
Multiple Anion CaptureSynthesis of Medium-Sized Lactones and Heterospirocyclic IsobenzofuranonesNNHn-BuLi (2 equiv)THF, 0 °CNNPhOPhNNOPhPh2 LiO2 LiClClO(COCl) 2OONNOPhPhLiClONNPhPhOOONNOOPhPh32% yield40% yield- COClOOClLewisacidAlCl 3 catalyzed: Naik, N. Synth. Commun. 1988, 18, 625.OOClClNNOO46% yieldPhPhLanger, P. Eur. J. Org. Chem. 2001, 1511-1517.Naik, N. Synth. Commun. 1988, 18, 625-632.
Multiple Anion CaptureSynthesis of Radialene-Shaped PyrrolesRadialenes are cyclic compounds containing only sp 2 -hybridized ring atoms and exocyclic double bonds.OOONMg 2+OMagnesidin2NNHn-BuLi (2 equiv)THF, 0 °CNNPh-CNNNNPh2 Li 2 LiTolNClNTolClNLanger, P.; Döring, M. Synlett. 1998, 399-401.Langer, P. Eur. J. Org. Chem. 2001, 2617-2627.PhNNTolNNHTolNot observed!
Multiple Anion CaptureSynthesis of Radialene-Shaped PyrrolesRadialenes are cyclic compounds containing only sp 2 -hybridized ring atoms and exocyclic double bonds.OOONMg 2+OMagnesidin2NNHn-BuLi (2 equiv)THF, 0 °CNNPh-CNNNNPh2 Li 2 LiRegioselectivity (C vs. N attack) proposed to be the product of relativeHOMO energies (AM1: C lower than N) as well as HSAB effects.TolNClNTolClTolNNNNClNTolR1,3-protonshiftTolNNNHNClNTolRNNTolNH 2 NRNTolLanger, P.; Döring, M. Synlett. 1998, 399-401.Langer, P. Eur. J. Org. Chem. 2001, 2617-2627.40% yield
Multiple Anion CaptureSynthesis of Radialene-Shaped PyrrolesRadialenes are cyclic compounds containing only sp 2 -hybridized ring atoms and exocyclic double bonds.OOONMg 2+OMagnesidin2NNHn-BuLi (2 equiv)THF, 0 °CNNPh-CNNNNPh2 Li 2 LiTolNClNTolClNNTolNRNCO 2 MeCO 2 MeOOMeMeOOtoluene, 80 °C- TolNH 2NNTolNRNHNHTolNNTolNH 2 NRNTol68% yieldLanger, P.; Döring, M. Synlett. 1998, 399-401.Langer, P. Eur. J. Org. Chem. 2001, 2617-2627.
Multiple Anion CaptureSynthesis of Nitrile Oligomers Using Allene Dianionsn-BuLiKOt-Bu2 MPhCNPhNNPh45% yieldBates, R. B. J. Org. Chem. 1979, 44, 2290-2291.Bates, R. B. J. Org. Chem. 1980, 45, 168-169.RPhPhOTBSLDA(3.3 equiv)-78 °CRPhPh·LiLi3-CR 4-CR 5-CRPh 0 12 51t-Bu 0 66 0R-CN(4.5 equiv)then H 2 OquenchRROHNR4-CRRRRNRHN3-CRRRNPhNNHPh HN5-CRRRRN4-Tol 0 28 03-Tol 40 0 traceLanger, P. Eur. J. Org. Chem. 2003, 1948-1953.
Multiple Anion CaptureSynthesis of Nitrile Oligomers Using Allene Dianionsn-BuLiKOt-Bu2 MPhCNPhNNPh45% yieldBates, R. B. J. Org. Chem. 1979, 44, 2290-2291.Bates, R. B. J. Org. Chem. 1980, 45, 168-169.RRRPhPh·LiLiR-CN(3 equiv)PhPhN·NNRRPhPhNNNRR- RCNPhPhN3-CRRNRRRRCNPhPhNNRRNRCNRPh Ph NPh PhNNNNNRRN RR N R4-CR5-CRLanger, P. Eur. J. Org. Chem. 2003, 1948-1953.
One-Pot Total SynthesisPoupon's Biomimetic Synthesis of NitraramineON· isolated from the desert shrub Nitraria schoberi foundin Asia, Australia, and the Middle EastNNitraramineH· exhibits "serotonin-like" activity in in vitro bioassays· thought to be the product of condensation of threeC 5 subunits derived from lysineNitraria schoberiNHOONHEtOH, reflux, 3 h (2-3% yield)- or -H 2 O, reflux, 3 h (0.5-1% yield)ONNHPoupon, E. Org. Lett. 2005, 7, 2497-2499.
One-Pot Total SynthesisPoupon's Biomimetic Synthesis of NitraramineON· isolated from the desert shrub Nitraria schoberi foundin Asia, Australia, and the Middle EastNNitraramineH· exhibits "serotonin-like" activity in in vitro bioassays· thought to be the product of condensation of threeC 5 subunits derived from lysineNitraria schoberi- H 2 OHNOHNNitraramine = C 15 H 24 N 2 O3% overall yield → 5 steps = 50% avg. yield / step→ 10 steps = 70% avg. yield / step→ 15 steps = 79% avg. yield / stepNHOONHNNOHNNOHHNNOONNHNitraramineNH- H 2 ONOPoupon, E. Org. Lett. 2005, 7, 2497-2499.
One-Pot Total SynthesisLiu's Synthesis of the Quinazolinone Fungal MetabolitesRONNRNHHNO= H, glyantrypine= Me, Fumiquinazoline F= i-Pr, Fiscalin B· Substance P (neurokinin) antagonists· Display analgesic / anti-inflammatorypropertiesONNR 2R 1NHpyrazino[2,1-b]quinazoline-3,6-dione coreOONNNHHNOFumiquinazoline GR 2 OHOH 2 NOOONONHNNHOOHNH 2anthranilic acidHOOR 1 NH 2NNHNOHNHAlantrypinoneGlyantrypine: Mantle, P. G. J. Chem. Soc. Perkin Trans. I 1992, 1495-1496.Fumiquinazoline F: Numata, A. Tetrahedron Lett. 1992, 33, 1621-1624.Fiscalin B: Cooper, R. J. Antibiot. 1993, 46, 545-553.Fumiquinazoline G: Numata, A. J. Chem. Soc. Perkin Trans. I 1995, 2345-2353.Alantrypinone: Larsen, T. O. J. Nat. Prod. 1998, 61, 1154-1157.Ardeemin: McAlpine, J. B. J. Antibiot. 1993, 46, 374-379.Ardeemin
One-Pot Total SynthesisLiu's Synthesis of the Quinazolinone Fungal MetabolitesL-amino acidD-amino acidONH 2OHHOOR 1NHBocP(OPh) 3 , pyrmicrowaveOONHBocNR 1benzoxazinoneMeOOR 2NH 2OONHNHR 2R 1ONHBocOMeONNR 2quinazolinoneCO 2 MeNHBocR 1ONNR 2R 1ONHpyrazino[2,1-b]quinazoline-3,6-dioneLiu, J.-F. Tetrahedron Lett. 2005, 46, 1241-1244.Liu, J.-F. J. Org. Chem. 2005, 70, 6339-6345.
One-Pot Total SynthesisLiu's Synthesis of the Quinazolinone Fungal MetabolitesONH 2OHHOONHBoc, P(OPh)3pyr, microwavethenOOMeONHNOGlyantrypineNHNH 2NNH(55% yield)OHOONHBocHNOHMeNH, P(OPh) 3pyr, microwavethenONH 2OMeONNMeNHOFumiquinazoline F(39% yield)OHOONHBocHNNH 2OHNH 2i-PrNH, P(OPh) 3pyr, microwavethenONH 2OMeONNNHOFiscalin B(20% yield)Liu, J.-F. Tetrahedron Lett. 2005, 46, 1241-1244.Liu, J.-F. J. Org. Chem. 2005, 70, 6339-6345.
One-Pot Total SynthesisLiu's Synthesis of the Quinazolinobenzodiazepine Fungal MetabolitesOOHOR 2 NH 2NH 2OHOOOOHNH 2HONHBoc, P(OPh)3pyr, microwaveNNNHOSclerotigenin(55% yield)2 equivOOHONHBocMe, P(OPh) 3pyr, microwaveNNMeNHOCircumdatin F(32% yield)HOONHBocCH 2 indole, P(OPh) 3pyr, microwaveONNNHOAsperlicin C(20% yield)Liu, J.-F. J. Org. Chem. 2005, 70, 10488-10493.Sclerotigenin: Gloer, J. B. J. Nat. Prod. 1999, 62, 650-652.Circumdatin F: Rahbæk, L. J. Nat. Prod. 1999, 62, 904-905.Asperlicin: Lotti, V. J. J. Antibiot. 1988, 41, 875-877.NH
<strong>Multicomponent</strong> <strong>Reactions</strong>Summary and ConclusionsAADBBAACCB· <strong>Multicomponent</strong> reactions are capable of condensing 3, 4, 5, 6, and even 7 reactant species in a single reaction mixture.· MCRs are underused in total synthesis. Though the most obvious applications lie in the realm of library synthesis, theextreme convergence afforded by these reactions provides a quick, efficient, and low-cost alternative to current linearsyntheses.Remember: In comparison to an analogous multi-step sequence, a low-yielding MCR is not as costly.· Most MCRs have a broad substrate scope capable of tolerating diverse functionality in addition to the reactive centers.This can set up MCR products for further cascade transformations.· Limitation: <strong>The</strong> more components that a MCR employs, the more complex the target it generates. <strong>The</strong> more complexthe target, the less generally applicable it becomes in total synthesis.· <strong>The</strong> library of known multicomponent reactions is far from complete! New combinations of existing reactions are alwayspossible and a firm understanding of reaction mechanism can lead to the discovery of novel modes of reactivity.AA + B + C + DBDC