Skin & Allergy News® - Global Academy for Medical Education
Skin & Allergy News® - Global Academy for Medical Education
Skin & Allergy News® - Global Academy for Medical Education
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
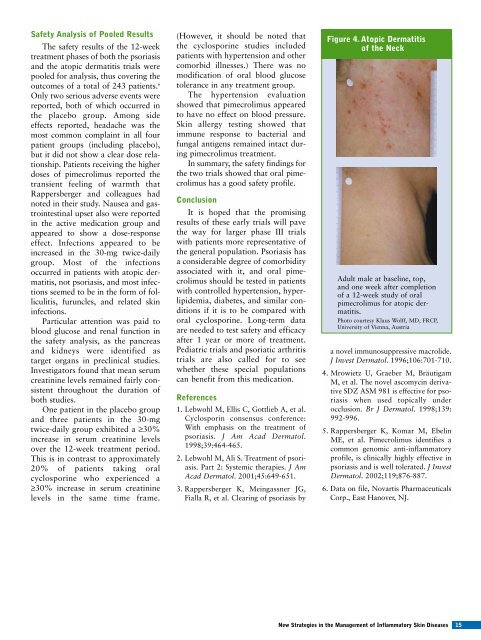
Safety Analysis of Pooled ResultsThe safety results of the 12-weektreatment phases of both the psoriasisand the atopic dermatitis trials werepooled <strong>for</strong> analysis, thus covering theoutcomes of a total of 243 patients. 6Only two serious adverse events werereported, both of which occurred inthe placebo group. Among sideeffects reported, headache was themost common complaint in all fourpatient groups (including placebo),but it did not show a clear dose relationship.Patients receiving the higherdoses of pimecrolimus reported thetransient feeling of warmth thatRappersberger and colleagues hadnoted in their study. Nausea and gastrointestinalupset also were reportedin the active medication group andappeared to show a dose-responseeffect. Infections appeared to beincreased in the 30-mg twice-dailygroup. Most of the infectionsoccurred in patients with atopic dermatitis,not psoriasis, and most infectionsseemed to be in the <strong>for</strong>m of folliculitis,furuncles, and related skininfections.Particular attention was paid toblood glucose and renal function inthe safety analysis, as the pancreasand kidneys were identified astarget organs in preclinical studies.Investigators found that mean serumcreatinine levels remained fairly consistentthroughout the duration ofboth studies.One patient in the placebo groupand three patients in the 30-mgtwice-daily group exhibited a ≥30%increase in serum creatinine levelsover the 12-week treatment period.This is in contrast to approximately20% of patients taking oralcyclosporine who experienced a≥30% increase in serum creatininelevels in the same time frame.(However, it should be noted thatthe cyclosporine studies includedpatients with hypertension and othercomorbid illnesses.) There was nomodification of oral blood glucosetolerance in any treatment group.The hypertension evaluationshowed that pimecrolimus appearedto have no effect on blood pressure.<strong>Skin</strong> allergy testing showed thatimmune response to bacterial andfungal antigens remained intact duringpimecrolimus treatment.In summary, the safety findings <strong>for</strong>the two trials showed that oral pimecrolimushas a good safety profile.ConclusionIt is hoped that the promisingresults of these early trials will pavethe way <strong>for</strong> larger phase III trialswith patients more representative ofthe general population. Psoriasis hasa considerable degree of comorbidityassociated with it, and oral pimecrolimusshould be tested in patientswith controlled hypertension, hyperlipidemia,diabetes, and similar conditionsif it is to be compared withoral cyclosporine. Long-term dataare needed to test safety and efficacyafter 1 year or more of treatment.Pediatric trials and psoriatic arthritistrials are also called <strong>for</strong> to seewhether these special populationscan benefit from this medication.References1. Lebwohl M, Ellis C, Gottlieb A, et al.Cyclosporin consensus conference:With emphasis on the treatment ofpsoriasis. J Am Acad Dermatol.1998;39:464-465.2. Lebwohl M, Ali S. Treatment of psoriasis.Part 2: Systemic therapies. J AmAcad Dermatol. 2001;45:649-651.3. Rappersberger K, Meingassner JG,Fialla R, et al. Clearing of psoriasis byFigure 4. Atopic Dermatitisof the NeckAdult male at baseline, top,and one week after completionof a 12-week study of oralpimecrolimus <strong>for</strong> atopic dermatitis.Photo courtesy Klaus Wolff, MD, FRCP,University of Vienna, Austriaa novel immunosuppressive macrolide.J Invest Dermatol. 1996;106:701-710.4. Mrowietz U, Graeber M, BräutigamM, et al. The novel ascomycin derivativeSDZ ASM 981 is effective <strong>for</strong> psoriasiswhen used topically underocclusion. Br J Dermatol. 1998;139:992-996.5. Rappersberger K, Komar M, EbelinME, et al. Pimecrolimus identifies acommon genomic anti-inflammatoryprofile, is clinically highly effective inpsoriasis and is well tolerated. J InvestDermatol. 2002;119;876-887.6. Data on file, Novartis PharmaceuticalsCorp., East Hanover, NJ.New Strategies in the Management of Inflammatory <strong>Skin</strong> Diseases 15