The essence of superconductivity and superconductors.
The essence of superconductivity and superconductors.
The essence of superconductivity and superconductors.
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
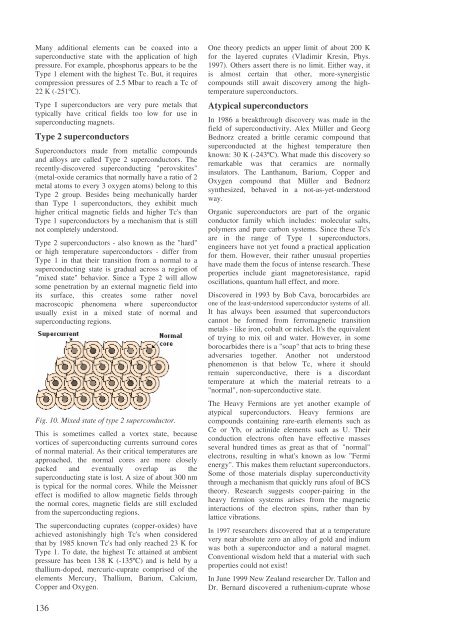
Many additional elements can be coaxed into asuperconductive state with the application <strong>of</strong> highpressure. For example, phosphorus appears to be theType 1 element with the highest Tc. But, it requirescompression pressures <strong>of</strong> 2.5 Mbar to reach a Tc <strong>of</strong>22 K (-251ºC).Type I <strong>superconductors</strong> are very pure metals thattypically have critical fields too low for use insuperconducting magnets.Type 2 <strong>superconductors</strong>Superconductors made from metallic compounds<strong>and</strong> alloys are called Type 2 <strong>superconductors</strong>. <strong>The</strong>recently-discovered superconducting "perovskites"(metal-oxide ceramics that normally have a ratio <strong>of</strong> 2metal atoms to every 3 oxygen atoms) belong to thisType 2 group. Besides being mechanically harderthan Type 1 <strong>superconductors</strong>, they exhibit muchhigher critical magnetic fields <strong>and</strong> higher Tc's thanType 1 <strong>superconductors</strong> by a mechanism that is stillnot completely understood.Type 2 <strong>superconductors</strong> - also known as the "hard"or high temperature <strong>superconductors</strong> - differ fromType 1 in that their transition from a normal to asuperconducting state is gradual across a region <strong>of</strong>"mixed state" behavior. Since a Type 2 will allowsome penetration by an external magnetic field intoits surface, this creates some rather novelmacroscopic phenomena where superconductorusually exist in a mixed state <strong>of</strong> normal <strong>and</strong>superconducting regions.Fig. 10. Mixed state <strong>of</strong> type 2 superconductor.This is sometimes called a vortex state, becausevortices <strong>of</strong> superconducting currents surround cores<strong>of</strong> normal material. As their critical temperatures areapproached, the normal cores are more closelypacked <strong>and</strong> eventually overlap as thesuperconducting state is lost. A size <strong>of</strong> about 300 nmis typical for the normal cores. While the Meissnereffect is modified to allow magnetic fields throughthe normal cores, magnetic fields are still excludedfrom the superconducting regions.<strong>The</strong> superconducting cuprates (copper-oxides) haveachieved astonishingly high Tc's when consideredthat by 1985 known Tc's had only reached 23 K forType 1. To date, the highest Tc attained at ambientpressure has been 138 K (-135ºC) <strong>and</strong> is held by athallium-doped, mercuric-cuprate comprised <strong>of</strong> theelements Mercury, Thallium, Barium, Calcium,Copper <strong>and</strong> Oxygen.One theory predicts an upper limit <strong>of</strong> about 200 Kfor the layered cuprates (Vladimir Kresin, Phys.1997). Others assert there is no limit. Either way, itis almost certain that other, more-synergisticcompounds still await discovery among the hightemperature<strong>superconductors</strong>.Atypical <strong>superconductors</strong>In 1986 a breakthrough discovery was made in thefield <strong>of</strong> <strong>superconductivity</strong>. Alex Müller <strong>and</strong> GeorgBednorz created a brittle ceramic compound thatsuperconducted at the highest temperature thenknown: 30 K (-243ºC). What made this discovery soremarkable was that ceramics are normallyinsulators. <strong>The</strong> Lanthanum, Barium, Copper <strong>and</strong>Oxygen compound that Müller <strong>and</strong> Bednorzsynthesized, behaved in a not-as-yet-understoodway.Organic <strong>superconductors</strong> are part <strong>of</strong> the organicconductor family which includes: molecular salts,polymers <strong>and</strong> pure carbon systems. Since these Tc'sare in the range <strong>of</strong> Type 1 <strong>superconductors</strong>,engineers have not yet found a practical applicationfor them. However, their rather unusual propertieshave made them the focus <strong>of</strong> intense research. <strong>The</strong>seproperties include giant magnetoresistance, rapidoscillations, quantum hall effect, <strong>and</strong> more.Discovered in 1993 by Bob Cava, borocarbides areone <strong>of</strong> the least-understood superconductor systems <strong>of</strong> all.It has always been assumed that <strong>superconductors</strong>cannot be formed from ferromagnetic transitionmetals - like iron, cobalt or nickel. It's the equivalent<strong>of</strong> trying to mix oil <strong>and</strong> water. However, in someborocarbides there is a "soap" that acts to bring theseadversaries together. Another not understoodphenomenon is that below Tc, where it shouldremain superconductive, there is a discordanttemperature at which the material retreats to a"normal", non-superconductive state.<strong>The</strong> Heavy Fermions are yet another example <strong>of</strong>atypical <strong>superconductors</strong>. Heavy fermions arecompounds containing rare-earth elements such asCe or Yb, or actinide elements such as U. <strong>The</strong>irconduction electrons <strong>of</strong>ten have effective massesseveral hundred times as great as that <strong>of</strong> "normal"electrons, resulting in what's known as low "Fermienergy". This makes them reluctant <strong>superconductors</strong>.Some <strong>of</strong> those materials display <strong>superconductivity</strong>through a mechanism that quickly runs afoul <strong>of</strong> BCStheory. Research suggests cooper-pairing in theheavy fermion systems arises from the magneticinteractions <strong>of</strong> the electron spins, rather than bylattice vibrations.In 1997 researchers discovered that at a temperaturevery near absolute zero an alloy <strong>of</strong> gold <strong>and</strong> indiumwas both a superconductor <strong>and</strong> a natural magnet.Conventional wisdom held that a material with suchproperties could not exist!In June 1999 New Zeal<strong>and</strong> researcher Dr. Tallon <strong>and</strong>Dr. Bernard discovered a ruthenium-cuprate whose136