Inspection Checklist for ABSL-3 Laboratories - Select Agent Program
Inspection Checklist for ABSL-3 Laboratories - Select Agent Program
Inspection Checklist for ABSL-3 Laboratories - Select Agent Program
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
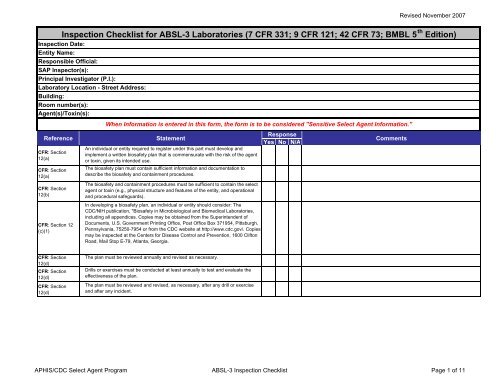
Revised November 2007<strong>Inspection</strong> <strong>Checklist</strong> <strong>for</strong> <strong>ABSL</strong>-3 <strong>Laboratories</strong> (7 CFR 331; 9 CFR 121; 42 CFR 73; BMBL 5 th Edition)<strong>Inspection</strong> Date:Entity Name:Responsible Official:SAP Inspector(s):Principal Investigator (P.I.):Laboratory Location - Street Address:Building:Room number(s):<strong>Agent</strong>(s)/Toxin(s):ReferenceCFR: Section12(a)CFR: Section12(a)CFR: Section12(b)CFR: Section 12(c)(1)When In<strong>for</strong>mation is entered in this <strong>for</strong>m, the <strong>for</strong>m is to be considered "Sensitive <strong>Select</strong> <strong>Agent</strong> In<strong>for</strong>mation."StatementAn individual or entity required to register under this part must develop andimplement a written biosafety plan that is commensurate with the risk of the agentor toxin, given its intended use.The biosafety plan must contain sufficient in<strong>for</strong>mation and documentation todescribe the biosafety and containment procedures.The biosafety and containment procedures must be sufficient to contain the selectagent or toxin (e.g., physical structure and features of the entity, and operationaland procedural safeguards).In developing a biosafety plan, an individual or entity should consider: TheCDC/NIH publication, "Biosafety in Microbiological and Biomedical <strong>Laboratories</strong>,including all appendices. Copies may be obtained from the Superintendent ofDocuments, U.S. Government Printing Office, Post Office Box 371954, Pittsburgh,Pennsylvania, 75250-7954 or from the CDC website at http://www.cdc.gov/. Copiesmay be inspected at the Centers <strong>for</strong> Disease Control and Prevention, 1600 CliftonRoad, Mail Stop E-79, Atlanta, Georgia.ResponseYes No N/ACommentsCFR: Section12(d)CFR: Section12(d)CFR: Section12(d)The plan must be reviewed annually and revised as necessary.Drills or exercises must be conducted at least annually to test and evaluate theeffectiveness of the plan.The plan must be reviewed and revised, as necessary, after any drill or exerciseand after any incident.APHIS/CDC <strong>Select</strong> <strong>Agent</strong> <strong>Program</strong> <strong>ABSL</strong>-3 <strong>Inspection</strong> <strong>Checklist</strong> Page 1 of 11
Revised November 2007ReferenceABMBL: A1StatementThe animal facility director establishes and en<strong>for</strong>ces policies, procedures, andprotocols <strong>for</strong> institutional policies and emergency situations. Each institute mustassure that worker safety and health concerns are addressed as part of the animalprotocol review. Prior to beginning a study animal protocols must also be reviewedand approved by the IACUC5 and the Institutional Biosafety Committee.ResponseYes No N/ACommentsBMBL: A2A safety manual specific to the animal facility is prepared or adopted inconsultation with the animal facility director and appropriate safetyprofessionals.BMBL: A2The safety manual must be available and accessible.BMBL: A2Personnel are advised of potential and special hazards, and are required to readand follow instructions on practices and procedures. Consideration should begiven to specific biohazards unique to the animal species and protocol in use.BMBL: A3Supervisor must ensure that animal care, laboratory and support personnel receiveappropriate training regarding their duties, animal husbandryprocedure, potential hazards, manipulations of infectious agents, necessaryprecautions to prevent hazard or exposures, and hazard/exposure evaluationprocedures (physical hazards, splashes, aerosolization, etc.).BMBL: A3BMBL: A3BMBL: A4BMBL: A4Personnel must receive annual updates or additional training when procedures orpolicies change.Records are maintained <strong>for</strong> all hazard evaluations, employee training sessions andstaff attendance.Appropriate medical surveillance program is in place, as determined by riskassessment.The need <strong>for</strong> an animal allergy prevention program should be considered. Facilitysupervisors should ensure that medical staff is in<strong>for</strong>med of potential occupationalhazards within the animal facility, to include those associated with research, animalhusbandry duties, animal care and manipulations.BMBL: A4Personal health status may impact an individual’s susceptibility to infection, abilityto receive immunizations or prophylactic interventions. There<strong>for</strong>e, all personneland particularly women of child-bearing age should be provided in<strong>for</strong>mationregarding immune competence and conditions that may predispose them toinfection. Individuals having these conditions should be encouraged to self-identifyto the institution’s healthcare provider <strong>for</strong> appropriate counseling and guidance.BMBL: A4BMBL: A5Personnel using respirators must be enrolled in an appropriately constitutedrespiratory protection program.A sign incorporating the universal biohazard symbol must be posted at theentrance to areas where infectious materials and/or animals are housed or aremanipulated.APHIS/CDC <strong>Select</strong> <strong>Agent</strong> <strong>Program</strong> <strong>ABSL</strong>-3 <strong>Inspection</strong> <strong>Checklist</strong> Page 2 of 11
Revised November 2007ReferenceBMBL: A5StatementThe sign must include the animal biosafety level, general occupational healthrequirements, personal protective equipment requirements, the supervisor’s name(or other responsible personnel), telephone number, and required procedures <strong>for</strong>entering and exiting the animal areas.ResponseYes No N/ACommentsBMBL: A5BMBL: A5BMBL: A5BMBL: A6Identification of specific infectious agents is recommended when more than oneagent is being used within an animal room.Security-sensitive agent in<strong>for</strong>mation and occupational health requirements shouldbe posted in accordance with the institutional policy.Advance consideration should be given to emergency and disaster recovery plans,as a contingency <strong>for</strong> man-made or natural disasters.Access to the animal room is limited to the fewest number of individuals possible.Only those persons required <strong>for</strong> program or support purposes are authorized toenter the animal facility and the areas where infectious materials and/or animalsare housed or are manipulated.BMBL: A6All persons including facility personnel, service workers, and visitors are advised ofthe potential hazards (natural or research pathogens, allergens, etc) and areinstructed on the appropriate safeguards.BMBL: A7BMBL: A7BMBL: A7Protective laboratory coats, gowns, or uni<strong>for</strong>ms are required to preventcontamination of personal clothing.Gloves are worn to prevent skin contact with contaminated, infectious/ andhazardous materials and when handling animals.Double-glove practices should be used when dictated by risk assessment.BMBL: A7Gloves and personal protective equipment should be removed in a manner thatminimizes transfer of infectious materials outside of the areas where infectiousmaterials and/or animals are housed or are manipulated.BMBL: A7Persons must wash their hands after removing gloves, and be<strong>for</strong>e leaving theareas where infectious materials and/or animals are housed or are manipulated.BMBL: A7BMBL: A8BMBL: A9BMBL: A10Eye and face and respiratory protection should be used in rooms containinginfected animals, as dictated by the risk assessment.Eating, drinking, smoking, handling contact lenses, applying cosmetics, and storingfood <strong>for</strong> human use should only be done in designated areas and are not permittedin animal or procedure rooms.All procedures are carefully per<strong>for</strong>med to minimize the creation of aerosols orsplatters of infectious materials and waste.Mouth pipetting is prohibited. Mechanical pipetting devices must be used.BMBL: A11BMBL: A11Policies <strong>for</strong> the safe handling of sharps, such as needles, scalpels, pipettes, andbroken glassware must be developed and implemented.When applicable, laboratory supervisors should adopt improved engineering andwork practice controls that reduce the risk of sharps injuries. Precautions,including those listed below, must always be taken with sharp items. Theseinclude:APHIS/CDC <strong>Select</strong> <strong>Agent</strong> <strong>Program</strong> <strong>ABSL</strong>-3 <strong>Inspection</strong> <strong>Checklist</strong> Page 3 of 11
Revised November 2007ReferenceBMBL: A11-aStatementNeedles and syringes or other sharp instruments are limited to use in the animalfacility when there is no alternative <strong>for</strong> such procedures as parenteral injection,blood collection, or aspiration of fluids from laboratory animals and diaphragmbottles.ResponseYes No N/ACommentsBMBL: A11-bDisposable needles must not be bent, sheared, broken, recapped, removed fromdisposable syringes, or otherwise manipulated by hand be<strong>for</strong>e disposal.BMBL: A11-bBMBL: A11-bUsed disposable needles must be carefully placed in puncture-resistant containersused <strong>for</strong> sharps disposal.Sharps containers should be located as close to the work site as possible.BMBL: A11-cNon-disposable sharps must be placed in a hard-walled container <strong>for</strong> transport to aprocessing area <strong>for</strong> decontamination, preferably by autoclaving.BMBL: A11-dBMBL: A11-dBroken glassware must not be handled directly; it should be removed using abrush and dustpan, tongs, or <strong>for</strong>ceps.Plasticware should be substituted <strong>for</strong> glassware whenever possible.BMBL: A11-eEquipment containing sharp edges and corners should be avoided.BMBL: A12Equipment and work surfaces are routinely decontaminated with an appropriatedisinfectant after work with an infectious agent, and after any spills, splashes, orother overt contamination.BMBL: A13Animals and plants not associated with the work being per<strong>for</strong>med must not bepermitted in the areas where infectious materials and/or animals are housed or aremanipulated.BMBL: A14BMBL: A15An effective integrated pest management program is required.All wastes from the animal room (including animal tissues, carcasses, andbedding) are transported from the animal room in leak-proof containers <strong>for</strong>appropriate disposal in compliance with applicable institutional, local and staterequirements.BMBL: A15BBMBL: B1Decontaminate all potentially infectious materials be<strong>for</strong>e disposal using an effectivemethod.Animal care staff, laboratory and routine support personnel must be provided amedical surveillance program as dictated by the risk assessment, andadministered appropriate immunizations <strong>for</strong> agents handled or potentially present,be<strong>for</strong>e entry into animal rooms.BMBL: B1When appropriate, a base line serum sample should be stored.BMBL: B2All procedures involving the manipulation of infectious materials, handling infectedanimals or the generations of aerosols must be conducted withinBSCs or other physical containment devices when practical.APHIS/CDC <strong>Select</strong> <strong>Agent</strong> <strong>Program</strong> <strong>ABSL</strong>-3 <strong>Inspection</strong> <strong>Checklist</strong> Page 4 of 11
Revised November 2007ReferenceBMBL: B2StatementWhen a procedure cannot be per<strong>for</strong>med within a biosafety cabinet, a combinationof personal protective equipment and other containment devices must be used.Consideration should be given to the use of restraint devices and practices thatreduce the risk of exposure during animal manipulations (e.g., physical restraintdevices, chemical restraint medications, etc).ResponseYes No N/ACommentsBMBL: B3The risk of infectious aerosols from infected animals or their bedding also can bereduced if animals are housed in containment caging systems (such as solid walland bottom cages covered with filter bonnets, open cages placed in inward flowventilated enclosures, HEPA-filter isolators and caging systems, or otherequivalent primary containment systems).BMBL: B4BMBL: B4BMBL: B4BMBL: B4BMBL: B5Actively ventilated caging systems must be designed to prevent the escape ofmicroorganisms from the cage.Exhaust plenums <strong>for</strong> these systems should be sealed to prevent escape ofmicroorganisms if the ventilation system becomes static, and the exhaust must beHEPA filtered.Safety mechanisms should be in place that prevent the cages and exhaustplenums from becoming positive to the surrounding area should the exhaust fanfail.The system should also be alarmed to indicate when operational malfunctionsoccur.A method <strong>for</strong> decontaminating all infectious materials must be available within thefacility, preferably within the areas where infectious materials and/or animals arehoused or are manipulated (e.g. autoclave, chemical disinfection, or otherapproved decontamination methods). Consideration should be given to means <strong>for</strong>decontaminating routine husbandry equipment, sensitive electronic and medicalequipment.BMBL: B5Decontaminate all potential infectious materials (including animal tissues,carcasses, contaminated bedding, unused feed, sharps, and other refuse) be<strong>for</strong>eremoval from the areas where infectious materials and/or animals are housed orare manipulated by an appropriate method. It is recommended that animalbedding and waste be decontaminated prior to manipulation and be<strong>for</strong>e removalfrom the areas where infectious materials and/or animals are housed or aremanipulated, preferably within the caging system.BMBL: B5Develop and implement an appropriate waste disposal program in compliance withapplicable institutional, local and state requirements. Autoclaving of content prior toincineration is recommended.BMBL: B6BMBL: B6BMBL: B6Equipment, cages, and racks should be handled in manner that minimizescontamination of other areas.Equipment must be decontaminated be<strong>for</strong>e repair, maintenance, or removal fromthe areas where infectious materials and/or animals are housed or aremanipulated.Spills involving infectious materials must be contained, decontaminated, andcleaned up by staff properly trained and equipped to work with infectious material.APHIS/CDC <strong>Select</strong> <strong>Agent</strong> <strong>Program</strong> <strong>ABSL</strong>-3 <strong>Inspection</strong> <strong>Checklist</strong> Page 5 of 11
ReferenceBMBL: B7BMBL: B7CBMBL: C1StatementIncidents that may result in exposure to infectious materials must be immediatelyevaluated and treated according to procedures described in the safety manual. Allsuch incidents must be reported to the animal facility supervisor or personneldesignated by the institution.Medical evaluation, surveillance, and treatment should be provided as appropriateand records maintained.Properly maintained BSCs, and other physical containment devices or equipment,should be used <strong>for</strong> all manipulations <strong>for</strong> infectious materials and when possible,animals. These manipulations include necropsy, harvesting of tissues or fluidsfrom infected animals or eggs, and intranasal inoculation of animals. The risk ofinfectious aerosols from infected animals or bedding can be reduced through theuse of primary barrier systems. These systems may include solid wall and bottomcages covered with filter bonnets; ventilated cage rack systems; or <strong>for</strong> larger cagesplaced in inward flow ventilated enclosures or other equivalent systems or devices.ResponseYes No N/ACommentsRevised November 2007BMBL: C2BMBL: C2BMBL: C2BMBL: C2BMBL: C2A risk assessment should determine the appropriate type of personal protectiveequipment to be utilized.Protective clothing such as uni<strong>for</strong>ms or scrub suits is worn by personnel within theanimal facility.Reusable clothing is appropriately contained and decontaminated be<strong>for</strong>e beinglaundered.Laboratory and protective clothing should never be taken home.Disposable personal protective equipment such as non-woven olefin cover-allsuits, wrap-around or solid-front gowns should be worn over this clothing, be<strong>for</strong>eentering the areas where infectious materials and/or animals are housed ormanipulated. Front-button laboratory coats are unsuitable.BMBL: C2BMBL: C2BMBL: C2BMBL: C3BMBL: C3BMBL: C3BMBL: C3Disposable personal protective equipment must be removed when leaving theareas where infectious materials and/or animals are housed or are manipulated.Scrub suits and uni<strong>for</strong>ms are removed be<strong>for</strong>e leaving the animal facility.Disposable personal protective equipment and other contaminated waste areappropriately contained and decontaminated prior to disposal.Appropriate eye, face and respiratory protection are worn by all personnel enteringareas where infectious materials and/or animals are housed or are manipulated.To prevent cross contamination boots, shoe covers, or other protective footwear,are used where indicated.Eye and face protection must be disposed of with other contaminated laboratorywaste or decontaminated be<strong>for</strong>e reuse.Persons who wear contact lenses should also wear eye protection when enteringareas with potentially high concentrations or airborne particulates.APHIS/CDC <strong>Select</strong> <strong>Agent</strong> <strong>Program</strong> <strong>ABSL</strong>-3 <strong>Inspection</strong> <strong>Checklist</strong> Page 6 of 11
Revised November 2007ReferenceBMBL: C4StatementGloves should be worn to protect hands from exposure to hazardous materials. Arisk assessment should be per<strong>for</strong>med to identify the appropriate glove <strong>for</strong> the taskand alternatives to latex gloves should be available. Procedures may require theuse of wearing two pairs of gloves (double-glove).ResponseYes No N/ACommentsBMBL: C4BMBL: C4Gloves are changed when contaminated, integrity has been compromised, or whenotherwise necessary.Gloves must not be worn outside the animal rooms.BMBL: C4BMBL: C4Gloves and personal protective equipment should be removed in a manner thatprohibits transfer of infectious materials.Do not wash or reuse disposable gloves.BMBL: C4Dispose of used gloves with other contaminated waste.BMBL: C4Persons must wash their hands after handling animals and be<strong>for</strong>e leaving theareas where infectious materials and/or animals are housed or are manipulated.BMBL: C4Hand washing should occur after the removal of gloves.DBMBL: D1BMBL: D1The animal facility is separated from areas that are open to unrestricted personneltraffic within the building.External facility doors are self-closing and self-locking.BMBL: D1Access to the animal facility is restricted.BMBL: D1Doors to areas where infectious materials and/or animals are housed or openinward, are self-closing, are kept closed when experimental animals are present,and should never be propped open.BMBL: D1BMBL: D1BMBL: D1Doors to cubicles inside an animal room may open outward or slide horizontally orvertically.Entry into the containment area is via a double-door entry which constitutes ananteroom/airlock and a change room.Showers may be considered based on risk assessment.BMBL: D1An additional double-door access anteroom or double-doored autoclave may beprovided <strong>for</strong> movement of supplies and wastes into and out of the facility.BMBL: D2A hand washing sink is located at the exit of the areas where infectious materialsand/or animals are housed or are manipulated. Additional sinks <strong>for</strong> hand washingshould be located in other appropriate locations within the facility.BMBL: D2The sink should be hands-free or automatically operated.BMBL: D2If the animal facility has multiple segregated areas where infectious materialsand/or animals are housed or are manipulated, a sink must also be available <strong>for</strong>hand washing at the exit from each segregated area.APHIS/CDC <strong>Select</strong> <strong>Agent</strong> <strong>Program</strong> <strong>ABSL</strong>-3 <strong>Inspection</strong> <strong>Checklist</strong> Page 7 of 11
Revised November 2007ReferenceBMBL: D2BMBL: D3BMBL: D3StatementSink traps are filled with water, and/or appropriate liquid to prevent the migration ofvermin and gases.The animal facility is designed, constructed, and maintained to facilitate cleaning,decontamination and housekeeping.The interior surfaces (walls, floors and ceilings) are water resistant.ResponseYes No N/ACommentsBMBL: D3Penetrations in floors, walls and ceiling surfaces are sealed, to include openingsaround ducts, doors and door frames, to facilitate pest control, proper cleaning anddecontamination.BMBL: D3Walls, floors and ceilings should <strong>for</strong>m a sealed and sanitizable surface.BMBL: D3Floors must be slip resistant, impervious to liquids, and resistant to chemicals.BMBL: D3Flooring is seamless, sealed resilient or poured floors, with integral cove bases.BMBL: D3Decontamination of an entire animal room should be considered when there hasbeen gross contamination of the space, significant changes in usage, <strong>for</strong> majorrenovations, or maintenance shut downs.BMBL: D3BMBL: D4BMBL: D4BMBL: D4<strong>Select</strong>ion of the appropriate materials and methods used to decontaminate theanimal room must be based on the risk assessment.Cabinets and bench tops must be impervious to water and resistant to heat,organic solvents, acids, alkalis, and other chemicals.Spaces between benches, cabinets, and equipment should be accessible <strong>for</strong>cleaning.Furniture should be minimized.BMBL: D4BMBL: D4Chairs used in animal area must be covered with a non-porous material that canbe easily cleaned and decontaminated.Furniture must be capable of supporting anticipated loads and uses.BMBL: D4Sharp edges and corners should be avoided.BMBL: D5BMBL: D5BMBL: D6BMBL: D6External windows are not recommended; if present, all windows must be sealedand must be resistant to breakage.The presence of windows may impact facility security and there<strong>for</strong>e should beassessed by security personnel.Ventilation to the facility should be provided in accordance with the Guide <strong>for</strong> Careand Use of Laboratory Animals.The direction of airflow into the animal facility is inward; animal rooms shouldmaintain inward directional airflow compared to adjoining hallways.BMBL: D6A ducted exhaust air ventilation system is provided.BMBL: D6Exhaust air is discharged to the outside without being recirculated to other rooms.This system creates directional airflow which draws air into the animal room from"clean" areas and toward "contaminated" areas.APHIS/CDC <strong>Select</strong> <strong>Agent</strong> <strong>Program</strong> <strong>ABSL</strong>-3 <strong>Inspection</strong> <strong>Checklist</strong> Page 8 of 11
Revised November 2007ReferenceBMBL: D6StatementVentilation system design should consider the heat and high moisture loadproduced during the cleaning of animal rooms and the cage wash process.ResponseYes No N/ACommentsBMBL: D6Filtration and other treatments of the exhaust air may not be required, but shouldbe considered based on site requirements, specific agent manipulations and useconditions.BMBL: D6BMBL: D6The exhaust must be dispersed away from occupied areas and air intakes, or theexhaust must be HEPA-filtered.Personnel must verify that the direction of the airflow (into the animal areas) isproper. It is recommended that a visual monitoring device that indicates andconfirms directional inward airflow be provided at the animal room entry.BMBL: D6BMBL: D6BMBL: D7BMBL: D8BMBL: D9Consideration should be given to installing an HVAC control system to preventsustained positive pressurization of the animal spaces.Audible alarms should be considered to notify personnel of ventilation and HVACsystem failure.Internal facility appurtenances, such as light fixtures, air ducts, and utility pipes, arearranged to minimize horizontal surface areas, to facilitate cleaning and minimizethe accumulation of debris or fomites.Floor drains must be maintained and filled with water, and/or appropriatedisinfectant to prevent the migration of vermin and gases.Cages are washed in a mechanical cage washer.BMBL: D9The mechanical cage washer has a final rinse temperature of at least 180°F.BMBL: D9BMBL: D9Cages should be autoclaved or otherwise decontaminated prior to removal from<strong>ABSL</strong>-3 space.The cage wash facility should be designed and constructed to accommodate highpressure spray systems, humidity, strong chemical disinfectants and 180°F watertemperatures, during the cage cleaning process.BMBL: D10BMBL: D11BMBL: D11BMBL: D11Illumination is adequate <strong>for</strong> all activities, avoiding reflections and glare that couldimpede vision.BSCs (Class II, Class III) must be installed so that fluctuations of the room airsupply and exhaust do not interfere with its proper operations.Class II BSCs should be located away from doors, heavily traveled laboratoryareas, and other possible airflow disruptions.HEPA filtered exhaust air from a Class II BSC can be safely re-circulated back intothe laboratory environment if the cabinet is tested and certified at least annuallyand operated according to manufacturer’s recommendations. BSCs can also beconnected to the laboratory exhaust system by either a thimble (canopy)connection or a direct (hard) connection.BMBL: D11BMBL: D11Provisions to assure proper safety cabinet per<strong>for</strong>mance and air system operationmust be verified.BSCs should be certified at least annually to assure correct per<strong>for</strong>mance.APHIS/CDC <strong>Select</strong> <strong>Agent</strong> <strong>Program</strong> <strong>ABSL</strong>-3 <strong>Inspection</strong> <strong>Checklist</strong> Page 9 of 11
Revised November 2007ReferenceBMBL: D11BMBL: D11StatementClass III BSCs must supply air in such a manner that prevents positivepressurization of the cabinet or the laboratory room.All BSCs should be used according to manufacturers’ recommendations.ResponseYes No N/ACommentsBMBL: D11When applicable, equipment that may produce infectious aerosols must becontained in devices that exhaust air through HEPA filtration or other equivalenttechnology be<strong>for</strong>e being discharged into the animal facility.BMBL: D11These HEPA filters should be tested and/or replaced at least annually.BMBL: D12An autoclave is available which is convenient to the animal rooms where thebiohazard is contained. The autoclave is utilized to decontaminate infectiousmaterials and waste be<strong>for</strong>e moving it to the other areas of the facility.BMBL: D12If not convenient to areas where infectious materials and/or animals are housed orare manipulated, special practices should be developed <strong>for</strong> transport of infectiousmaterials designated alternate locations within the facility.BMBL: D13BMBL: D14Emergency eyewash and shower are readily available; location is determined byrisk assessment.The <strong>ABSL</strong>-3 facility design and operational procedures must be documented.BMBL: D14BMBL: D14BMBL: D15The facility must be tested to verify that the design and operational parametershave been met prior to use.Facilities should be re-verified at least annually against these procedures asmodified by operational experience.Additional environmental protection (e.g., personnel showers, HEPA filtration ofexhaust air, containment of other piped services, and the provision or effluentdecontamination) should be considered if recommended by the agent summarystatement, as determined by risk assessment of the site conditions, or otherapplicable federal, state or local regulations.APHIS/CDC <strong>Select</strong> <strong>Agent</strong> <strong>Program</strong> <strong>ABSL</strong>-3 <strong>Inspection</strong> <strong>Checklist</strong> Page 10 of 11
ReferenceInspector summary and comments:StatementResponseYes No N/ACommentsRevised November 2007Recommendations:Inspector completing checklist:Other inspectors present:Date:Date:APHIS/CDC <strong>Select</strong> <strong>Agent</strong> <strong>Program</strong> <strong>ABSL</strong>-3 <strong>Inspection</strong> <strong>Checklist</strong> Page 11 of 11