Exam 3 Solutions
Exam 3 Solutions
Exam 3 Solutions
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
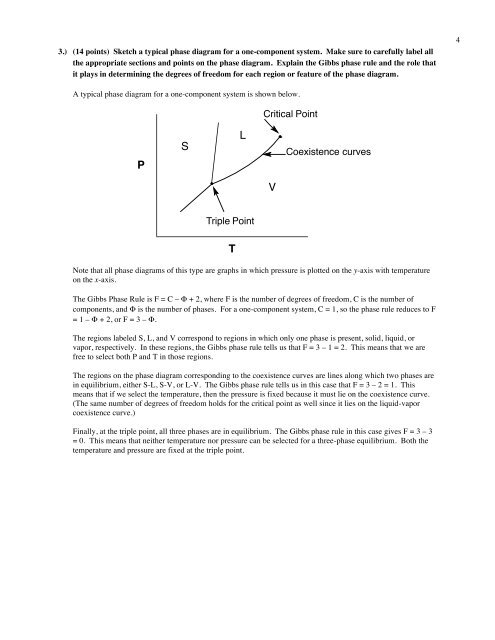
3.) (14 points) Sketch a typical phase diagram for a one-component system. Make sure to carefully label allthe appropriate sections and points on the phase diagram. Explain the Gibbs phase rule and the role thatit plays in determining the degrees of freedom for each region or feature of the phase diagram.4A typical phase diagram for a one-component system is shown below.Critical PointPSLCoexistence curvesVTriple PointTNote that all phase diagrams of this type are graphs in which pressure is plotted on the y-axis with temperatureon the x-axis.The Gibbs Phase Rule is F = C – Φ + 2, where F is the number of degrees of freedom, C is the number ofcomponents, and Φ is the number of phases. For a one-component system, C = 1, so the phase rule reduces to F= 1 – Φ + 2, or F = 3 – Φ.The regions labeled S, L, and V correspond to regions in which only one phase is present, solid, liquid, orvapor, respectively. In these regions, the Gibbs phase rule tells us that F = 3 – 1 = 2. This means that we arefree to select both P and T in those regions.The regions on the phase diagram corresponding to the coexistence curves are lines along which two phases arein equilibrium, either S-L, S-V, or L-V. The Gibbs phase rule tells us in this case that F = 3 – 2 = 1. Thismeans that if we select the temperature, then the pressure is fixed because it must lie on the coexistence curve.(The same number of degrees of freedom holds for the critical point as well since it lies on the liquid-vaporcoexistence curve.)Finally, at the triple point, all three phases are in equilibrium. The Gibbs phase rule in this case gives F = 3 – 3= 0. This means that neither temperature nor pressure can be selected for a three-phase equilibrium. Both thetemperature and pressure are fixed at the triple point.