Timed ur<strong>in</strong>e collections are labour-<strong>in</strong>tensive and notoriouslyunreliable. As a result many equations for estimat<strong>in</strong>g creat<strong>in</strong><strong>in</strong>eclearance have been derived that only need measurement ofserum creat<strong>in</strong><strong>in</strong>e. The most widely recognised of these is theCockcroft-Gault formula, which relies on patient age, weight,gender and serum creat<strong>in</strong><strong>in</strong>e.creat<strong>in</strong><strong>in</strong>eclearance(mL/m<strong>in</strong>)The accuracy of this formula for estimat<strong>in</strong>g creat<strong>in</strong><strong>in</strong>e clearanceis equivalent to that from a timed ur<strong>in</strong>e collection, so there isno good reason for us<strong>in</strong>g a 24-hour collection. Manufacturers'<strong>renal</strong> dos<strong>in</strong>g recommendations for medications are based onCockcroft-Gault estimates of <strong>renal</strong> function, so this formula isalso recommended when estimat<strong>in</strong>g creat<strong>in</strong><strong>in</strong>e clearance for thepurpose of calculat<strong>in</strong>g drug doses that vary accord<strong>in</strong>g to <strong>renal</strong>function.=(140 – age) x lean body weight (kg) (x 0.85 for females)Cl<strong>in</strong>icians should be aware of some important limitations of theCockcroft-Gault estimation of <strong>renal</strong> function. It is:■ not validated <strong>in</strong> some populations■ unreliable <strong>in</strong> extremes of body size (that is, <strong>in</strong> severemalnutrition or obesity)■ imprecise and unreliable for rapidly chang<strong>in</strong>g <strong>renal</strong> function(for example <strong>in</strong>tensive care, acute <strong>renal</strong> failure).What is estimated GFR?<strong>Australian</strong> pathology laboratories have started rout<strong>in</strong>ely<strong>in</strong>clud<strong>in</strong>g an estimated GFR (eGFR) <strong>in</strong> all biochemistry reportsthat <strong>in</strong>clude serum creat<strong>in</strong><strong>in</strong>e. The report<strong>in</strong>g of serum creat<strong>in</strong><strong>in</strong>ehas also been standardised to be <strong>in</strong> micromol/L (so the actualnumber is 1000 times that when reported as mmol/L).The formula used to calculate eGFR was derived as part of alarge study of the effect of dietary prote<strong>in</strong> restriction on theprogression of <strong>renal</strong> failure. (This was the Modification of Diet <strong>in</strong>Renal Disease study, hence the MDRD formula. 1 ) The advantageof this formula is that it does not require knowledge of thepatient's height or weight as the eGFR is calculated us<strong>in</strong>g serumcreat<strong>in</strong><strong>in</strong>e, age and gender.It is crucial that cl<strong>in</strong>icians realise that the eGFR is not estimat<strong>in</strong>gthe patient's actual GFR, but is estimat<strong>in</strong>g an adjusted GFR –which assumes that the patient is of average body size. Thisexpla<strong>in</strong>s how the number can be calculated without anyknowledge of the patient's actual size. Average body sizeequates to a body surface area of 1.73 m 2 , and so the eGFR isreported as mL/m<strong>in</strong>/1.73 m 2 . In practice, this means that whileone person who is twice the size of another, of the same age,gender and serum creat<strong>in</strong><strong>in</strong>e, will have twice the actual GFR, theeGFR for both will be the same.serum creat<strong>in</strong><strong>in</strong>e (micromol/L) x 0.815The eGFR is primarily <strong>in</strong>tended to be a screen<strong>in</strong>g tool for <strong>renal</strong><strong>disease</strong> <strong>in</strong> the community, <strong>in</strong> association with other signs of<strong>renal</strong> <strong>disease</strong> such as ur<strong>in</strong>ary abnormalities and hypertension.It has similar limitations as the Cockcroft-Gault equation 2 ,<strong>in</strong>clud<strong>in</strong>g that it is not validated <strong>in</strong> Aborig<strong>in</strong>al and Torres StraitIslander people.eGFR is not preferred for calculat<strong>in</strong>g drug dosesDrug dos<strong>in</strong>g should be based on the patient's actual GFR andnot an adjusted GFR. While recognis<strong>in</strong>g that the Cockcroft-Gaultequation has limitations, it does at least take <strong>in</strong>to account bodysize when estimat<strong>in</strong>g GFR, whereas the eGFR does not. Us<strong>in</strong>gthe eGFR to calculate dosages would lead to overdos<strong>in</strong>g ofsmall patients and underdos<strong>in</strong>g of large patients. Overdos<strong>in</strong>g<strong>in</strong>creases the risk of toxicity of drugs with a narrow therapeuticrange, while underdos<strong>in</strong>g reduces efficacy. The MDRD formulaused to calculate eGFR can be manipulated to adjust for apatient's body surface area (if the patient's height and weightare known). A recently published observational analysissuggests wide variation between the formulas. 3 However, asyet it is unknown whether the MDRD formula is superior toCockcroft-Gault for calculat<strong>in</strong>g drug doses.<strong>Prescrib<strong>in</strong>g</strong> for dialysis patientsFor the purpose of drug prescrib<strong>in</strong>g, patients on dialysis(haemodialysis or peritoneal dialysis) should be consideredto have a creat<strong>in</strong><strong>in</strong>e clearance/GFR of less than 10 mL/m<strong>in</strong>.Certa<strong>in</strong> drugs are actively removed from the circulation dur<strong>in</strong>gdialysis, and this needs to be considered when decid<strong>in</strong>g on thetim<strong>in</strong>g of adm<strong>in</strong>istration as well as the dosage. Factors thatmay reduce the extent to which a drug is dialysed <strong>in</strong>clude largemolecular size of the drug, high prote<strong>in</strong> b<strong>in</strong>d<strong>in</strong>g, large volumeof distribution and high lipid solubility. In addition to theseparameters, the type of dialyser membrane may also affectdrug clearance, as will blood and dialysate flow rates. If a drugis known to be dialysed, patients hav<strong>in</strong>g haemodialysis may be<strong>in</strong>structed to take the drug after the dialysis session.Dose alteration <strong>in</strong> <strong>renal</strong> impairmentOnce <strong>renal</strong> impairment has been detected and creat<strong>in</strong><strong>in</strong>eclearance estimated, the need for dose alteration of <strong>renal</strong>lycleared drugs must be determ<strong>in</strong>ed. Generally dose adjustmentis needed when the creat<strong>in</strong><strong>in</strong>e clearance is below 60 mL/m<strong>in</strong>.People who have been tak<strong>in</strong>g a drug for many years may needa dose adjustment as they age. Adjustments can be achieved bya reduction <strong>in</strong> dose, or an extension of the dos<strong>in</strong>g <strong>in</strong>terval, orboth. Knowledge of appropriate dosage adjustment is importantto ensure the drug is effective and that accumulation and furtherkidney damage is avoided. There are various references toconsult <strong>in</strong> Australia <strong>in</strong>clud<strong>in</strong>g the approved product <strong>in</strong>formationand the <strong>Australian</strong> Medic<strong>in</strong>es Handbook. International references<strong>in</strong>clude the Renal Drug Handbook and Drug prescrib<strong>in</strong>g <strong>in</strong> <strong>renal</strong>failure. 4 Table 1 lists some of the commonly prescribed drugsthat require dose alteration <strong>in</strong> <strong>renal</strong> impairment.18 | VOLUME 30 | NUMBER 1 | FEBRUARY 2007
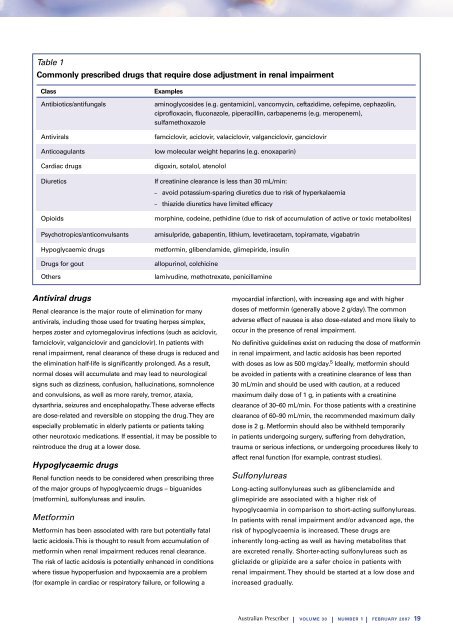
Table 1Commonly prescribed drugs that require dose adjustment <strong>in</strong> <strong>renal</strong> impairmentClassAntibiotics/antifungalsAntiviralsAnticoagulantsCardiac drugsDiureticsOpioidsPsychotropics/anticonvulsantsHypoglycaemic drugsDrugs for goutOthersExamplesam<strong>in</strong>oglycosides (e.g. gentamic<strong>in</strong>), vancomyc<strong>in</strong>, ceftazidime, cefepime, cephazol<strong>in</strong>,ciprofloxac<strong>in</strong>, fluconazole, piperacill<strong>in</strong>, carbapenems (e.g. meropenem),sulfamethoxazolefamciclovir, aciclovir, valaciclovir, valganciclovir, ganciclovirlow molecular weight hepar<strong>in</strong>s (e.g. enoxapar<strong>in</strong>)digox<strong>in</strong>, sotalol, atenololIf creat<strong>in</strong><strong>in</strong>e clearance is less than 30 mL/m<strong>in</strong>:– avoid potassium-spar<strong>in</strong>g diuretics due to risk of hyperkalaemia– thiazide diuretics have limited efficacymorph<strong>in</strong>e, code<strong>in</strong>e, pethid<strong>in</strong>e (due to risk of accumulation of active or toxic metabolites)amisulpride, gabapent<strong>in</strong>, lithium, levetiracetam, topiramate, vigabatr<strong>in</strong>metform<strong>in</strong>, glibenclamide, glimepiride, <strong>in</strong>sul<strong>in</strong>allopur<strong>in</strong>ol, colchic<strong>in</strong>elamivud<strong>in</strong>e, methotrexate, penicillam<strong>in</strong>eAntiviral drugsRenal clearance is the major route of elim<strong>in</strong>ation for manyantivirals, <strong>in</strong>clud<strong>in</strong>g those used for treat<strong>in</strong>g herpes simplex,herpes zoster and cytomegalovirus <strong>in</strong>fections (such as aciclovir,famciclovir, valganciclovir and ganciclovir). In patients with<strong>renal</strong> impairment, <strong>renal</strong> clearance of these drugs is reduced andthe elim<strong>in</strong>ation half-life is significantly prolonged. As a result,normal doses will accumulate and may lead to neurologicalsigns such as dizz<strong>in</strong>ess, confusion, halluc<strong>in</strong>ations, somnolenceand convulsions, as well as more rarely, tremor, ataxia,dysarthria, seizures and encephalopathy. These adverse effectsare dose-related and reversible on stopp<strong>in</strong>g the drug. They areespecially problematic <strong>in</strong> elderly patients or patients tak<strong>in</strong>gother neurotoxic medications. If essential, it may be possible tore<strong>in</strong>troduce the drug at a lower dose.Hypoglycaemic drugsRenal function needs to be considered when prescrib<strong>in</strong>g threeof the major groups of hypoglycaemic drugs – biguanides(metform<strong>in</strong>), sulfonylureas and <strong>in</strong>sul<strong>in</strong>.Metform<strong>in</strong>Metform<strong>in</strong> has been associated with rare but potentially fatallactic acidosis. This is thought to result from accumulation ofmetform<strong>in</strong> when <strong>renal</strong> impairment reduces <strong>renal</strong> clearance.The risk of lactic acidosis is potentially enhanced <strong>in</strong> conditionswhere tissue hypoperfusion and hypoxaemia are a problem(for example <strong>in</strong> cardiac or respiratory failure, or follow<strong>in</strong>g amyocardial <strong>in</strong>farction), with <strong>in</strong>creas<strong>in</strong>g age and with higherdoses of metform<strong>in</strong> (generally above 2 g/day). The commonadverse effect of nausea is also dose-related and more likely tooccur <strong>in</strong> the presence of <strong>renal</strong> impairment.No def<strong>in</strong>itive guidel<strong>in</strong>es exist on reduc<strong>in</strong>g the dose of metform<strong>in</strong><strong>in</strong> <strong>renal</strong> impairment, and lactic acidosis has been reportedwith doses as low as 500 mg/day. 5 Ideally, metform<strong>in</strong> shouldbe avoided <strong>in</strong> patients with a creat<strong>in</strong><strong>in</strong>e clearance of less than30 mL/m<strong>in</strong> and should be used with caution, at a reducedmaximum daily dose of 1 g, <strong>in</strong> patients with a creat<strong>in</strong><strong>in</strong>eclearance of 30–60 mL/m<strong>in</strong>. For those patients with a creat<strong>in</strong><strong>in</strong>eclearance of 60–90 mL/m<strong>in</strong>, the recommended maximum dailydose is 2 g. Metform<strong>in</strong> should also be withheld temporarily<strong>in</strong> patients undergo<strong>in</strong>g surgery, suffer<strong>in</strong>g from dehydration,trauma or serious <strong>in</strong>fections, or undergo<strong>in</strong>g procedures likely toaffect <strong>renal</strong> function (for example, contrast studies).SulfonylureasLong-act<strong>in</strong>g sulfonylureas such as glibenclamide andglimepiride are associated with a higher risk ofhypoglycaemia <strong>in</strong> comparison to short-act<strong>in</strong>g sulfonylureas.In patients with <strong>renal</strong> impairment and/or advanced age, therisk of hypoglycaemia is <strong>in</strong>creased. These drugs are<strong>in</strong>herently long-act<strong>in</strong>g as well as hav<strong>in</strong>g metabolites thatare excreted <strong>renal</strong>ly. Shorter-act<strong>in</strong>g sulfonylureas such asgliclazide or glipizide are a safer choice <strong>in</strong> patients with<strong>renal</strong> impairment. They should be started at a low dose and<strong>in</strong>creased gradually.| VOLUME 30 | NUMBER 1 | FEBRUARY 2007 19