PTI-609: A Novel Analgesic that Binds Filamin A to Control Opioid ...
PTI-609: A Novel Analgesic that Binds Filamin A to Control Opioid ...
PTI-609: A Novel Analgesic that Binds Filamin A to Control Opioid ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
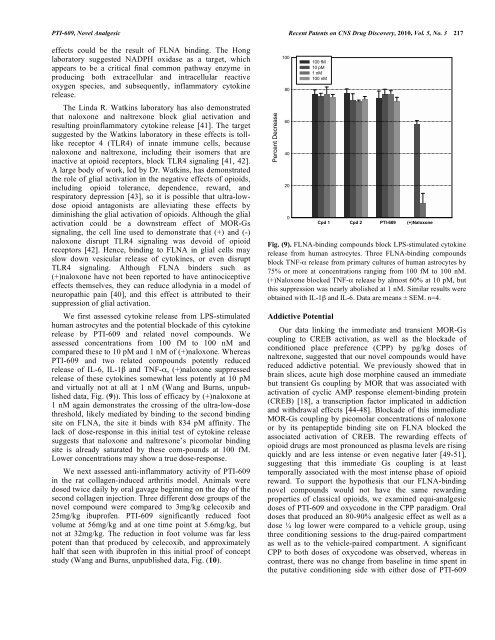
<strong>PTI</strong>-<strong>609</strong>, <strong>Novel</strong> <strong>Analgesic</strong> Recent Patents on CNS Drug Discovery, 2010, Vol. 5, No. 3 217effects could be the result of FLNA binding. The Honglabora<strong>to</strong>ry suggested NADPH oxidase as a target, whichappears <strong>to</strong> be a critical final common pathway enzyme inproducing both extracellular and intracellular reactiveoxygen species, and subsequently, inflamma<strong>to</strong>ry cy<strong>to</strong>kinerelease.The Linda R. Watkins labora<strong>to</strong>ry has also demonstrated<strong>that</strong> naloxone and naltrexone block glial activation andresulting proinflamma<strong>to</strong>ry cy<strong>to</strong>kine release [41]. The targetsuggested by the Watkins labora<strong>to</strong>ry in these effects is <strong>to</strong>lllikerecep<strong>to</strong>r 4 (TLR4) of innate immune cells, becausenaloxone and naltrexone, including their isomers <strong>that</strong> areinactive at opioid recep<strong>to</strong>rs, block TLR4 signaling [41, 42].A large body of work, led by Dr. Watkins, has demonstratedthe role of glial activation in the negative effects of opioids,including opioid <strong>to</strong>lerance, dependence, reward, andrespira<strong>to</strong>ry depression [43], so it is possible <strong>that</strong> ultra-lowdoseopioid antagonists are alleviating these effects bydiminishing the glial activation of opioids. Although the glialactivation could be a downstream effect of MOR-Gssignaling, the cell line used <strong>to</strong> demonstrate <strong>that</strong> (+) and (-)naloxone disrupt TLR4 signaling was devoid of opioidrecep<strong>to</strong>rs [42]. Hence, binding <strong>to</strong> FLNA in glial cells mayslow down vesicular release of cy<strong>to</strong>kines, or even disruptTLR4 signaling. Although FLNA binders such as(+)naloxone have not been reported <strong>to</strong> have antinociceptiveeffects themselves, they can reduce allodynia in a model ofneuropathic pain [40], and this effect is attributed <strong>to</strong> theirsuppression of glial activation.We first assessed cy<strong>to</strong>kine release from LPS-stimulatedhuman astrocytes and the potential blockade of this cy<strong>to</strong>kinerelease by <strong>PTI</strong>-<strong>609</strong> and related novel compounds. Weassessed concentrations from 100 fM <strong>to</strong> 100 nM andcompared these <strong>to</strong> 10 pM and 1 nM of (+)naloxone. Whereas<strong>PTI</strong>-<strong>609</strong> and two related compounds potently reducedrelease of IL-6, IL-1 and TNF-, (+)naloxone suppressedrelease of these cy<strong>to</strong>kines somewhat less potently at 10 pMand virtually not at all at 1 nM (Wang and Burns, unpublisheddata, Fig. (9)). This loss of efficacy by (+)naloxone at1 nM again demonstrates the crossing of the ultra-low-dosethreshold, likely mediated by binding <strong>to</strong> the second bindingsite on FLNA, the site it binds with 834 pM affinity. Thelack of dose-response in this initial test of cy<strong>to</strong>kine releasesuggests <strong>that</strong> naloxone and naltrexone’s picomolar bindingsite is already saturated by these com-pounds at 100 fM.Lower concentrations may show a true dose-response.We next assessed anti-inflamma<strong>to</strong>ry activity of <strong>PTI</strong>-<strong>609</strong>in the rat collagen-induced arthritis model. Animals weredosed twice daily by oral gavage beginning on the day of thesecond collagen injection. Three different dose groups of thenovel compound were compared <strong>to</strong> 3mg/kg celecoxib and25mg/kg ibuprofen. <strong>PTI</strong>-<strong>609</strong> significantly reduced footvolume at 56mg/kg and at one time point at 5.6mg/kg, butnot at 32mg/kg. The reduction in foot volume was far lesspotent than <strong>that</strong> produced by celecoxib, and approximatelyhalf <strong>that</strong> seen with ibuprofen in this initial proof of conceptstudy (Wang and Burns, unpublished data, Fig. (10).Percent Decrease100806040200100 fM10 pM1 nM100 nMCpd 1 Cpd 2 <strong>PTI</strong>-<strong>609</strong> (+)NaloxoneFig. (9). FLNA-binding compounds block LPS-stimulated cy<strong>to</strong>kinerelease from human astrocytes. Three FLNA-binding compoundsblock TNF- release from primary cultures of human astrocytes by75% or more at concentrations ranging from 100 fM <strong>to</strong> 100 nM.(+)Naloxone blocked TNF- release by almost 60% at 10 pM, butthis suppression was nearly abolished at 1 nM. Similar results wereobtained with IL-1 and IL-6. Data are means ± SEM. n=4.Addictive PotentialOur data linking the immediate and transient MOR-Gscoupling <strong>to</strong> CREB activation, as well as the blockade ofconditioned place preference (CPP) by pg/kg doses ofnaltrexone, suggested <strong>that</strong> our novel compounds would havereduced addictive potential. We previously showed <strong>that</strong> inbrain slices, acute high dose morphine caused an immediatebut transient Gs coupling by MOR <strong>that</strong> was associated withactivation of cyclic AMP response element-binding protein(CREB) [18], a transcription fac<strong>to</strong>r implicated in addictionand withdrawal effects [44-48]. Blockade of this immediateMOR-Gs coupling by picomolar concentrations of naloxoneor by its pentapeptide binding site on FLNA blocked theassociated activation of CREB. The rewarding effects ofopioid drugs are most pronounced as plasma levels are risingquickly and are less intense or even negative later [49-51],suggesting <strong>that</strong> this immediate Gs coupling is at leasttemporally associated with the most intense phase of opioidreward. To support the hypothesis <strong>that</strong> our FLNA-bindingnovel compounds would not have the same rewardingproperties of classical opioids, we examined equi-analgesicdoses of <strong>PTI</strong>-<strong>609</strong> and oxycodone in the CPP paradigm. Oraldoses <strong>that</strong> produced an 80-90% analgesic effect as well as adose log lower were compared <strong>to</strong> a vehicle group, usingthree conditioning sessions <strong>to</strong> the drug-paired compartmentas well as <strong>to</strong> the vehicle-paired compartment. A significantCPP <strong>to</strong> both doses of oxycodone was observed, whereas incontrast, there was no change from baseline in time spent inthe putative conditioning side with either dose of <strong>PTI</strong>-<strong>609</strong>