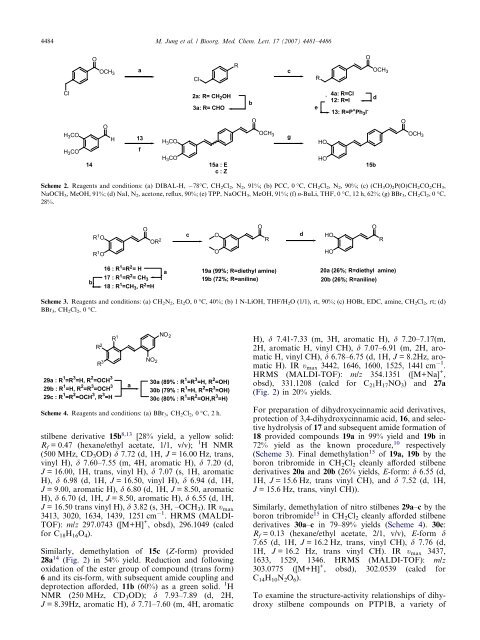
4484 M. Jung et al. / Bioorg. Med. Chem. Lett. 17 (2007) 4481–4486OOCH 3aClRcROOCH 3ClH 3 COH 3 CO14OH13fH 3 COH 3 CO2a: R= CH 2 OH3a: R= CHO15a : Ec:ZbOOCH 3geHOHO4a: R=Cl12: R=I13: R=P + Ph 3 I -d15bOOCH 3Scheme 2. Reagents <str<strong>on</strong>g>and</str<strong>on</strong>g> c<strong>on</strong>diti<strong>on</strong>s: (a) DIBAL-H, 78°C, CH 2 Cl 2 ,N 2 , 91%; (b) PCC, 0 °C, CH 2 Cl 2 ,N 2 , 90%; (c) (CH 3 O) 2 P(O)CH 2 CO 2 CH 3 ,NaOCH 3 , MeOH, 91%; (d) NaI, N 2 , acet<strong>on</strong>e, reflux, 90%; (e) TPP, NaOCH 3 , MeOH, 91%; (f) n-BuLi, THF, 0 °C, 12 h, 62%; (g) BBr 3 ,CH 2 Cl 2 ,0°C,28%.R 1 OOOR 2cOORdHOORR 1 OOHOb16 : R 1 =R 2 = H17 : R 1 =R 2 = CH 318 : R 1 =CH 3 , R 2 =Ha19a (99%; R=diethyl amine)19b (72%; R=aniline)20a (26%; R=diethyl amine)20b (26%; R=aniline)Scheme 3. Reagents <str<strong>on</strong>g>and</str<strong>on</strong>g> c<strong>on</strong>diti<strong>on</strong>s: (a) CH 2 N 2 ,Et 2 O, 0 °C, 40%; (b) 1 N-LiOH, THF/H 2 O (1/1), rt, 90%; (c) HOBt, EDC, amine, CH 2 Cl 2 , rt; (d)BBr 3 ,CH 2 Cl 2 ,0°C.R 2R 329a : R 1 =R 3 =H, R 2 =OCH 329b : R 1 =H, R 2 =R 3 =OCH 329c : R 1 =R 2 =OCH 3 , R 3 =HR 1aNO 2NO 230a (89% : R 1 =R 3 =H, R 2 =OH)30b (79% : R 1 =H, R 2 =R 3 =OH)30c (80% : R 1 =R 2 =OH,R 3 =H)Scheme 4. Reagents <str<strong>on</strong>g>and</str<strong>on</strong>g> c<strong>on</strong>diti<strong>on</strong>s: (a) BBr 3 ,CH 2 Cl 2 ,0°C, 2 h.<str<strong>on</strong>g>stilbene</str<strong>on</strong>g> derivative 15b 8,13 [28% yield, a yellow solid:R f = 0.47 (hexane/ethyl acetate, 1/1, v/v);1 H NMR(500 MHz, CD 3 OD) d 7.72 (d, 1H, J = 16.00 Hz, trans,vinyl H), d 7.60–7.55 (m, 4H, aromatic H), d 7.20 (d,J = 16.00, 1H, trans, vinyl H), d 7.07 (s, 1H, aromaticH), d 6.98 (d, 1H, J = 16.50, vinyl H), d 6.94 (d, 1H,J = 9.00, aromatic H), d 6.80 (d, 1H, J = 8.50, aromaticH), d 6.70 (d, 1H, J = 8.50, aromatic H), d 6.55 (d, 1H,J = 16.50 trans vinyl H), d 3.82 (s, 3H, –OCH 3 ). IR t max3413, 3020, 1634, 1439, 1251 cm 1 . HRMS (MALDI-TOF): m/z 297.0743 ([M+H] + , obsd), 296.1049 (calcdfor C 18 H 16 O 4 ).Similarly, demethylati<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> 15c (Z-form) provided28a 14 (Fig. 2) in 54% yield. Reducti<strong>on</strong> <str<strong>on</strong>g>and</str<strong>on</strong>g> followingoxidati<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> the ester group <str<strong>on</strong>g>of</str<strong>on</strong>g> compound (trans form)6 <str<strong>on</strong>g>and</str<strong>on</strong>g> its cis-form, with subsequent amide coupling <str<strong>on</strong>g>and</str<strong>on</strong>g>deprotecti<strong>on</strong> afforded, 11b (60%) as a green solid. 1 HNMR (250 MHz, CD 3 OD); d 7.93–7.89 (d, 2H,J = 8.39Hz, aromatic H), d 7.71–7.60 (m, 4H, aromaticH), d 7.41-7.33 (m, 3H, aromatic H), d 7.20–7.17(m,2H, aromatic H, vinyl CH), d 7.07–6.91 (m, 2H, aromaticH, vinyl CH), d 6.78–6.75 (d, 1H, J = 8.2Hz, aromaticH). IR t max 3442, 1646, 1600, 1525, 1441 cm 1 .HRMS (MALDI-TOF): m/z 354.1351 ([M+Na] + ,obsd), 331.1208 (calcd for C 21 H 17 NO 3 ) <str<strong>on</strong>g>and</str<strong>on</strong>g> 27a(Fig. 2) in 20% yields.For preparati<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> dihydroxycinnamic acid <str<strong>on</strong>g>derivatives</str<strong>on</strong>g>,protecti<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> 3,4-dihydroxycinnamic acid, 16, <str<strong>on</strong>g>and</str<strong>on</strong>g> selectivehydrolysis <str<strong>on</strong>g>of</str<strong>on</strong>g> 17 <str<strong>on</strong>g>and</str<strong>on</strong>g> subsequent amide formati<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g>18 provided compounds 19a in 99% yield <str<strong>on</strong>g>and</str<strong>on</strong>g> 19b in72% yield as the known procedure, 10 respectively(Scheme 3). Final demethylati<strong>on</strong> 15 <str<strong>on</strong>g>of</str<strong>on</strong>g> 19a, 19b by thebor<strong>on</strong> tribromide in CH 2 Cl 2 cleanly afforded <str<strong>on</strong>g>stilbene</str<strong>on</strong>g><str<strong>on</strong>g>derivatives</str<strong>on</strong>g> 20a <str<strong>on</strong>g>and</str<strong>on</strong>g> 20b (26% yields, E-form: d 6.55 (d,1H, J = 15.6 Hz, trans vinyl CH), <str<strong>on</strong>g>and</str<strong>on</strong>g> d 7.52 (d, 1H,J = 15.6 Hz, trans, vinyl CH)).Similarly, demethylati<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> nitro <str<strong>on</strong>g>stilbene</str<strong>on</strong>g>s 29a–c by thebor<strong>on</strong> tribromide 15 in CH 2 Cl 2 cleanly afforded <str<strong>on</strong>g>stilbene</str<strong>on</strong>g><str<strong>on</strong>g>derivatives</str<strong>on</strong>g> 30a–c in 79–89% yields (Scheme 4). 30c:R f = 0.13 (hexane/ethyl acetate, 2/1, v/v), E-form d7.65 (d, 1H, J = 16.2 Hz, trans, vinyl CH), d 7.76 (d,1H, J = 16.2 Hz, trans vinyl CH). IR t max 3437,1633, 1529, 1346. HRMS (MALDI-TOF): m/z303.0775 ([M+H] + , obsd), 302.0539 (calcd forC 14 H 10 N 2 O 6 ).To examine the structure-activity relati<strong>on</strong>ships <str<strong>on</strong>g>of</str<strong>on</strong>g> dihydroxy<str<strong>on</strong>g>stilbene</str<strong>on</strong>g> compounds <strong>on</strong> PTP1B, a variety <str<strong>on</strong>g>of</str<strong>on</strong>g>
M. Jung et al. / Bioorg. Med. Chem. Lett. 17 (2007) 4481–4486 4485Table 1. Inhibiti<strong>on</strong> against PTP1B <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>stilbene</str<strong>on</strong>g> <str<strong>on</strong>g>derivatives</str<strong>on</strong>g> aCompoundIC 50 (lM)7 25.67a— c9 26011a 10111b 27.215a— c15b 14.916 — c20a— c20b— c26a— c26b 137.527a— c27b 35.428a— c30a— c30b 19030c 16831 (cis-<str<strong>on</strong>g>stilbene</str<strong>on</strong>g>) 17 — c32 (curcumin) 18 — cMolybdate b 21RK-682 b,19 45DMSO— ca IC 50 values are means <str<strong>on</strong>g>and</str<strong>on</strong>g> SD <str<strong>on</strong>g>of</str<strong>on</strong>g> three experiments.b Positive c<strong>on</strong>trol(Na 2 MoO 4 Æ2H 2 O).c Inactive.trans-<str<strong>on</strong>g>stilbene</str<strong>on</strong>g> compounds with two hydroxy groups <strong>on</strong>phenyl rings <str<strong>on</strong>g>of</str<strong>on</strong>g> the parent <str<strong>on</strong>g>stilbene</str<strong>on</strong>g> skelet<strong>on</strong> were synthesized<str<strong>on</strong>g>and</str<strong>on</strong>g> the inhibitory activity <strong>on</strong> PTP1B was determined.16 All E- <str<strong>on</strong>g>and</str<strong>on</strong>g> Z-form were separated by usingcolumn silica gel <str<strong>on</strong>g>and</str<strong>on</strong>g> bioassay was performed as a singleregioisomer form. Using the colorimetric assay <str<strong>on</strong>g>based</str<strong>on</strong>g> <strong>on</strong>the rate <str<strong>on</strong>g>of</str<strong>on</strong>g> formati<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> p-nitrophenolate i<strong>on</strong> indicator,PTP1B activity was assessed in the presence <str<strong>on</strong>g>and</str<strong>on</strong>g> absence<str<strong>on</strong>g>of</str<strong>on</strong>g> the indicated compounds. IC 50 values were calculated<str<strong>on</strong>g>based</str<strong>on</strong>g> <strong>on</strong> the method <str<strong>on</strong>g>of</str<strong>on</strong>g> Burke et al. 16 All measurementsare means <str<strong>on</strong>g>and</str<strong>on</strong>g> SD <str<strong>on</strong>g>of</str<strong>on</strong>g> n observati<strong>on</strong>s, with a typical variability<str<strong>on</strong>g>of</str<strong>on</strong>g> 10% am<strong>on</strong>g observati<strong>on</strong>s. As a positive c<strong>on</strong>trol,the effects <str<strong>on</strong>g>of</str<strong>on</strong>g> the known inhibitors, molybdate<str<strong>on</strong>g>and</str<strong>on</strong>g> RK-682, were also evaluated, <str<strong>on</strong>g>and</str<strong>on</strong>g> the IC 50 valueswere found to fall within literature values. As shownin Table 1, 3,4-dimethoxy trans-<str<strong>on</strong>g>stilbene</str<strong>on</strong>g>s (7a, 15a, <str<strong>on</strong>g>and</str<strong>on</strong>g>26a) with protecti<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> 3,4-dihydroxy groups had noinhibitory effects. This observati<strong>on</strong> supports the idea,predicted by molecular modeling (Fig. 1), that protecti<strong>on</strong>with methyl group <str<strong>on</strong>g>of</str<strong>on</strong>g> the 3,4-dihydroxy groups <strong>on</strong>phenyl rings inhibits the formati<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> the stable radicalnecessary for inhibiti<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> PTP1B. 3,4-Dihydroxy <str<strong>on</strong>g>stilbene</str<strong>on</strong>g>s(7 <str<strong>on</strong>g>and</str<strong>on</strong>g> 15b) with a methoxy carb<strong>on</strong>yl <strong>on</strong> the sec<strong>on</strong>dphenyl ring showed the best <str<strong>on</strong>g>and</str<strong>on</strong>g> nearly complete inhibiti<strong>on</strong><str<strong>on</strong>g>of</str<strong>on</strong>g> PTP1B <str<strong>on</strong>g>and</str<strong>on</strong>g> had more potency than either 3,4-dihydroxy trans-<str<strong>on</strong>g>stilbene</str<strong>on</strong>g> (11a) with4 0 -aldehyde groupor 3,4-dihydroxy trans-<str<strong>on</strong>g>stilbene</str<strong>on</strong>g> (9) with 4 0 -alcoholgroup. Extensi<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> the c<strong>on</strong>jugated system <str<strong>on</strong>g>of</str<strong>on</strong>g> 7 to 15bfurther increased potency <str<strong>on</strong>g>of</str<strong>on</strong>g> PTP1B inhibiti<strong>on</strong>. The nitro<str<strong>on</strong>g>stilbene</str<strong>on</strong>g> compounds (30a) with <strong>on</strong>ly <strong>on</strong>e hydroxygroup at C-3 positi<strong>on</strong> <strong>on</strong> the phenyl ring <str<strong>on</strong>g>of</str<strong>on</strong>g> the parent<str<strong>on</strong>g>stilbene</str<strong>on</strong>g> skelet<strong>on</strong> had no inhibitory effects. However,the inhibitory potency was markedly enhanced by introducinganother hydroxy group at C-2 or C-4 positi<strong>on</strong>sto phenyl ring (30b <str<strong>on</strong>g>and</str<strong>on</strong>g> 30c), <str<strong>on</strong>g>of</str<strong>on</strong>g> the parent <str<strong>on</strong>g>stilbene</str<strong>on</strong>g> skelet<strong>on</strong>,thus indicating the importance <str<strong>on</strong>g>of</str<strong>on</strong>g> a hydroxy functi<strong>on</strong>at the C-2 or C-4 positi<strong>on</strong>s. Furthermore, additi<strong>on</strong><str<strong>on</strong>g>of</str<strong>on</strong>g> two phenolic hydroxy groups <str<strong>on</strong>g>of</str<strong>on</strong>g> cis-<str<strong>on</strong>g>stilbene</str<strong>on</strong>g> (31) to3,4-dihydroxy<str<strong>on</strong>g>stilbene</str<strong>on</strong>g> (26b) markedly increased theinhibitory activity. The inhibiti<strong>on</strong> by 30b <str<strong>on</strong>g>and</str<strong>on</strong>g> 30c was<str<strong>on</strong>g>of</str<strong>on</strong>g> nearly the same magnitude, with perhaps the presence<str<strong>on</strong>g>of</str<strong>on</strong>g> the hydroxy group at positi<strong>on</strong> C-4 having an effect <strong>on</strong>potency. However, 3,4-dihydroxycinnamic acid 16 <str<strong>on</strong>g>and</str<strong>on</strong>g>its amide <str<strong>on</strong>g>derivatives</str<strong>on</strong>g>, 20a <str<strong>on</strong>g>and</str<strong>on</strong>g> 20b, show no inhibiti<strong>on</strong>,suggesting necessity <str<strong>on</strong>g>of</str<strong>on</strong>g> the sec<strong>on</strong>d phenyl ring c<strong>on</strong>nectedthrough the double b<strong>on</strong>d for PTP1B inhibiti<strong>on</strong>.On the basis <str<strong>on</strong>g>of</str<strong>on</strong>g> these results, it appeared that the 3,4-dihydroxy motif at the parent phenyl ring <str<strong>on</strong>g>and</str<strong>on</strong>g> 2 0 ,4 0 -electr<strong>on</strong> withdrawing groups such as ester (7, 15b),aldehyde (11a), nitro groups (30b <str<strong>on</strong>g>and</str<strong>on</strong>g> 30c) <str<strong>on</strong>g>and</str<strong>on</strong>g> amides(11b, 27b) 20 <str<strong>on</strong>g>of</str<strong>on</strong>g> trans <str<strong>on</strong>g>stilbene</str<strong>on</strong>g> analogs enhance inhibitoryactivity <str<strong>on</strong>g>of</str<strong>on</strong>g> PTP1B. It is noteworthy that trans<str<strong>on</strong>g>derivatives</str<strong>on</strong>g> (15b <str<strong>on</strong>g>and</str<strong>on</strong>g> 27b) are more relatively potentthan cis <str<strong>on</strong>g>derivatives</str<strong>on</strong>g> (28a <str<strong>on</strong>g>and</str<strong>on</strong>g> 27a) probably due tobinding suitability with PTP1B. Especially, the amine<str<strong>on</strong>g>derivatives</str<strong>on</strong>g> (28b) for future testing are expected to havehigher antioxidant effect than the ester <str<strong>on</strong>g>derivatives</str<strong>on</strong>g>because the l<strong>on</strong>e pair <str<strong>on</strong>g>of</str<strong>on</strong>g> electr<strong>on</strong>s located at the nitrogenatom <str<strong>on</strong>g>of</str<strong>on</strong>g> the amide group can be c<strong>on</strong>jugated withp-b<strong>on</strong>d system better than that <str<strong>on</strong>g>of</str<strong>on</strong>g> the oxygen atom<str<strong>on</strong>g>of</str<strong>on</strong>g> the ester group. 20Evaluati<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> antioxidant activity <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>stilbene</str<strong>on</strong>g> <str<strong>on</strong>g>derivatives</str<strong>on</strong>g>7a, 9, 15a, 15b, 30b with xanthine oxidase assay revealedthat there is no direct correlati<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> radical scavengingeffect with PTP1B inhibiti<strong>on</strong>.In c<strong>on</strong>clusi<strong>on</strong>, we designed, synthesized, <str<strong>on</strong>g>and</str<strong>on</strong>g> developednovel <str<strong>on</strong>g>stilbene</str<strong>on</strong>g> <str<strong>on</strong>g>derivatives</str<strong>on</strong>g> as potential protein tyrosinephosphatase 1B inhibitors. Am<strong>on</strong>g them, 7, 11b, 15b,<str<strong>on</strong>g>and</str<strong>on</strong>g> 27b showed str<strong>on</strong>g inhibitory activities with IC 50values ranging from 14.95 to 35.4 lM against thePTP1B enzyme. Particularly, compound 7 shows inhibitoryactivity comparable to that <str<strong>on</strong>g>of</str<strong>on</strong>g> molybdate, while15b shows a 3-fold lower IC 50 than the clinically studiedRK682. Compound 15b deserves further evaluati<strong>on</strong> as apossible type-2 antidiabetic drug c<str<strong>on</strong>g>and</str<strong>on</strong>g>idate <str<strong>on</strong>g>based</str<strong>on</strong>g> <strong>on</strong> themechanism <str<strong>on</strong>g>of</str<strong>on</strong>g> PTP1B inhibiti<strong>on</strong>. This result suggeststhat introducti<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> electr<strong>on</strong> withdrawing groups (7),amide groups (11b, 27b), or extensi<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> the c<strong>on</strong>jugati<strong>on</strong>(15b) into the <str<strong>on</strong>g>stilbene</str<strong>on</strong>g> molecule may stabilize the generatedradicals.AcknowledgmentsThis research was supported by a research grant fromthe Ministry <str<strong>on</strong>g>of</str<strong>on</strong>g> Health & Welfare (Project No.B040002), Republic <str<strong>on</strong>g>of</str<strong>on</strong>g> Korea. We wish to express ourthanks to Pr<str<strong>on</strong>g>of</str<strong>on</strong>g>essor J<strong>on</strong>gsun Kim, Y<strong>on</strong>sei UniversityCollege <str<strong>on</strong>g>of</str<strong>on</strong>g> Medicine, for initial PTP1B screening, <str<strong>on</strong>g>and</str<strong>on</strong>g>Bioinformatics & Molecular <str<strong>on</strong>g>Design</str<strong>on</strong>g> Research Center(BMDRC) Y<strong>on</strong>sei University for drug design. Y.L.,E.L., M.S., D.L., <str<strong>on</strong>g>and</str<strong>on</strong>g> N.P. acknowledge the fellowship<str<strong>on</strong>g>of</str<strong>on</strong>g> the BK 21 program from the Ministry <str<strong>on</strong>g>of</str<strong>on</strong>g> Educati<strong>on</strong><str<strong>on</strong>g>and</str<strong>on</strong>g> Human Resources Development.