Medical Necessity Guidelines: Genetic Testing ... - Tufts Health Plan
Medical Necessity Guidelines: Genetic Testing ... - Tufts Health Plan
Medical Necessity Guidelines: Genetic Testing ... - Tufts Health Plan
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
G. The Member, of any age, has triple negative breast cancer. Breast cancer is defined as triple negative breast cancer when thetumor that does not have receptors for any of the following; estrogen, progesterone or human epidermal growth factorreceptor 2 (HER2).Note: For male members, multi-site BRCA3 test will be approved if Ashkenazic. If not Ashkenazic, a full panel BRCA1&2 will beapproved. If there is a known mutation, single-site testing will be approved.<strong>Tufts</strong> <strong>Health</strong> <strong>Plan</strong> may authorize single site analysis only for members who have a 1 st degree relative with a known BRCA 1or 2mutation, regardless of personal history or descent.For members who are of a partial Ashkenazic or non-Ashkenazic descent if the member meets the Ashkenazic criteria above andthe family history of breast and/or ovarian cancer occurred predominantly in non-Ashkenazic relatives, <strong>Tufts</strong> <strong>Health</strong> <strong>Plan</strong> mayauthorize full panel BRCA1 & BRCA2 genetic testing.For members who are of Ashkenazic descent who have a negative multi-site BRCA3 test AND who have a personal history ofbreast OR ovarian cancer, <strong>Tufts</strong> <strong>Health</strong> <strong>Plan</strong> may authorize full panel BRCA1 & BRCA2 genetic testing (reflex testing).All testing must be performed at a contracting laboratory facility when available.COVERAGE GUIDELINES FOR BART TM TESTINGLarge genomic rearrangements occur in a small percentage (
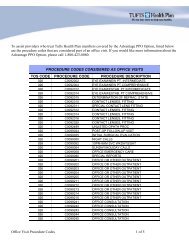
Code Description81217 BRCA2 (breast cancer 2) (eg, hereditary breast and ovarian cancer) gene analysis; known familial variantREFERENCES1. Bergthorsson, J.T., Ejlertsen, B., Olsen, J.H., Borg, A., Nielsen, K.V. et al. BRCA1 and BRCA2 mutation status and cancer familyhistory of Danish women affected with multifocal or bilateral breast cancer at a young age. Journal of <strong>Medical</strong> <strong>Genetic</strong>s. 2001;38(6):361.2. Berliner, J., Fay, A. Risk assessment and genetic counseling for hereditary breast and ovarian cancer: recommendations of theNational Society of <strong>Genetic</strong> Counselors. Journal of <strong>Genetic</strong> Counseling. 2007;16: 241-2603. De Sanjose, S., Leone, M., Berez, V. et al. Prevalence of BRCA1 and BRCA2 germline mutations in young breast cancerpatients: a population-based study. International Journal of Cancer. 2003;106:588-593.4. Frank, T.S., Deffenbaugh, A.M., Reid, J.E. et al. Clinical characteristics of individuals with germline mutations in BRCA1 andBRCA2: analysis of 10,000 individuals. Journal of Clinical Oncology. 2002; 20(6):1480-1490.5. Gene Reviews. BRCA1 and BRCA2 Hereditary Breast/Ovarian Cancer. Updated December 12, 2005. Accessed on December 29,2006 at: http://www.genetests.org/query?dz=brca16. German Consortium for Hereditary Breast and Ovarian Cancer. Comprehensive analysis of 989 patients with breast or ovariancancer provides BRCA1 and BRCA2 mutation profiles and frequencies for the German population. International Journal ofCancer. 2002; 97:472-480.7. Hendrickson, B, Judkins, T., et al. Recurrent intragenic rearrangement mutations in the tumor suppressor gene BRCA1:prevalence results from 12,272 patients at high risk for breast and/or ovarian cancers and method of biochemical analysis.Poster Presentation. American Society of Clinical Oncology. Annual Meeting. 2004.8. Myriad <strong>Genetic</strong> Laboratories. BRACAnalysis® Rearrangement Test resource Guide. Accessed on October 1, 2009 at:http://myriadresourceguide.com/pdfs/Myriad-Resource-Guide-BART-Criteria.pdf9. National Comprehensive Cancer Network. What is triple negative breast cancer? Accessed on May 1, 2012 at:http://www.nccn.com/component/content/article/57-cancer-answers/242-cancer-answers-triple-negative-breastcancer.html10. Pal, T., Permuth-Way, J., Betts, J. et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinomacases. Cancer. December 15, 2005; 104(12).11. Steinmann, D., Bremer, M., Rades, D. et al. Mutations of the BRCA1 and BRCA2 genes in patients with bilateral breast cancer.British Journal of Cancer. 2001; 85(6):850-858.12. U.S. Preventive Services Task Force (USPSTF). <strong>Genetic</strong> risk assessment and BRCA mutation testing for breast and ovariancancer susceptibility: recommendation statement. Annals of Internal Med. 2005; 143(5):355-361.13. U.S. Preventive Services Task Force (USPSTF) [website]. <strong>Genetic</strong> risk assessment and BRCA mutation testing for breast andovarian cancer susceptibility. Summary of Recommendations. September 2005. Retrieved on October 28, 2006 fromhttp://www.ahrq.gov/clinic/uspstf/uspsbrgen.htm.14. Weitzel, J., Lagos, V., Cullinane, C. et al. Limited family structure and BRCA gene mutation status in single cases of breastcancer. Journal of American <strong>Medical</strong> Association. June 20, 2007; 297(23): 2587-2595.APPROVAL HISTORY January 23, 2004: Reviewed by the Clinical Coverage Criteria Committee February 27, 2004: Criteria for the authorization of multi-site BRCA3 test for female members of Ashkenazi descent changedfrom requiring personal history of two primary breast cancers, each diagnosed before age 50, to personal history of a singleprimary breast cancer diagnosed before age 50. April 1, 2004: D. 6. a. Criteria for authorization of full panel BRCA 1 & BRCA 2 genetic test for female members who are not ofAshkenazi descent and have the following risk factor was changed to reflect that both relatives need to be on the same sideof the family to receive authorization for testing with no personal history of breast or ovarian cancer. April 15, 2005: Criteria expanded to cover single site BRCA1 and BRCA 2 testing for members who have a 1 st degree relativewith a known mutation in BRCA1or BRCA2, coverage for testing when a male relative has been diagnosed with breast cancerat any age, and the addition of criteria to cover Large Rearrangement Panel testing for members who meet the criteria fortesting, tested negative and were tested prior to August 12, 2004. March 10, 2006: Several clarifications and additions were made: Definition of high risk as greater than ten percent risk of apositive test result added, use of the BRCAPRO and MYRIAD Models in determining member’s risk added, diagnosis of ductalcancer in situ as synonymous with a breast cancer diagnosis explained. Change D.6.b.: to include non-Ashkenazic memberswith two or more 1 st or 2 nd degree relatives with a diagnosis of ovarian cancer at any age. January 1, 2007: Several clarifications were made to coverage for members of Ashkenazi descent, BART testing was added toLimitations.3 <strong>Medical</strong> <strong>Necessity</strong> <strong>Guidelines</strong>:Multi-site BRCA3, Single Site BRCA1 or BRCA2, & BART
November 13, 2007: Criteria for coverage clarified in cases where members have 1 st degree relatives with known BRCA 1orBRCA2 mutation.January 30, 2008: Criteria for coverage of BRCA testing for females, not of Ashkenazi descent, changed: Personal of history ofprimary breast cancer changed from before age 40 to before age 50. Definitions of first and second degree relatives added toOverview.February 11, 2009: For consideration, BRCAPRO calculation must be submitted by the requesting provider which shows thatthe calculated risk of the Member having the mutation is greater than ten percentOctober 7, 2009 for an effective date of November 1, 2009: Coverage guidelines for BART testing added to the guideline.December 2009: limitations moved: placed within the body of the criteria.February 1, 2010: Reviewed by <strong>Medical</strong> Policy Advisory Group Committee (MPAGC), no content changes. Administrativeprocess changed.May 2011: Reviewed by MSPAC. BRCAPro risk clarified to read; “≥ (greater than or equal to).”January 1, 2012: New CPT codes addedApril 1, 2012: Coding update, HCPCS codes deleted by CMSMay 9, 2012: Added coverage of testing when the Member has a tumor which is triple negativeBACKGROUND, PRODUCT AND DISCLAIMER INFORMATION<strong>Medical</strong> <strong>Necessity</strong> <strong>Guidelines</strong> are developed to determine coverage for <strong>Tufts</strong> <strong>Health</strong> <strong>Plan</strong> benefits, and are published to provide abetter understanding of the basis upon which coverage decisions are made. <strong>Tufts</strong> <strong>Health</strong> <strong>Plan</strong> makes coverage decisions usingthese guidelines, along with the Member’s benefit document, and in coordination with the Member’s physician(s) on a case-bycasebasis considering the individual Member's health care needs.<strong>Medical</strong> <strong>Necessity</strong> <strong>Guidelines</strong> are developed for selected therapeutic or diagnostic services found to be safe, but proven effectivein a limited, defined population of patients or clinical circumstances. They include concise clinical coverage criteria based oncurrent literature review, consultation with practicing physicians in the <strong>Tufts</strong> <strong>Health</strong> <strong>Plan</strong> service area who are medical experts inthe particular field, FDA and other government agency policies, and standards adopted by national accreditation organizations.<strong>Tufts</strong> <strong>Health</strong> <strong>Plan</strong> revises and updates <strong>Medical</strong> <strong>Necessity</strong> <strong>Guidelines</strong> annually, or more frequently if new evidence becomesavailable that suggests needed revisions.<strong>Medical</strong> <strong>Necessity</strong> <strong>Guidelines</strong> apply to all fully insured <strong>Tufts</strong> <strong>Health</strong> <strong>Plan</strong> products unless otherwise noted in this guideline or theMember’s benefit document. This guideline does not apply to <strong>Tufts</strong> <strong>Health</strong> <strong>Plan</strong> Medicare Preferred or to certain delegated servicearrangements. For self-insured plans, coverage may vary depending on the terms of the benefit document. If a discrepancy existsbetween a <strong>Medical</strong> <strong>Necessity</strong> Guideline and a self-insured Member’s benefit document, the provisions of the benefit documentwill govern. Applicable state or federal mandates will take precedence. Providers in the New Hampshire service area are subject toCigna’s provider agreements with respect to CareLink SM members.Treating providers are solely responsible for the medical advice and treatment of Members. The use of this guideline is not aguarantee of payment or a final prediction of how specific claim(s) will be adjudicated. Claims payment is subject to eligibility andbenefits on the date of service, coordination of benefits, referral/authorization, utilization management guidelines whenapplicable, and adherence to plan policies, plan procedures, and claims editing logic.Provider Services4 <strong>Medical</strong> <strong>Necessity</strong> <strong>Guidelines</strong>:Multi-site BRCA3, Single Site BRCA1 or BRCA2, & BART