Chemical Compatibility Table (pdf)
Chemical Compatibility Table (pdf)
Chemical Compatibility Table (pdf)
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
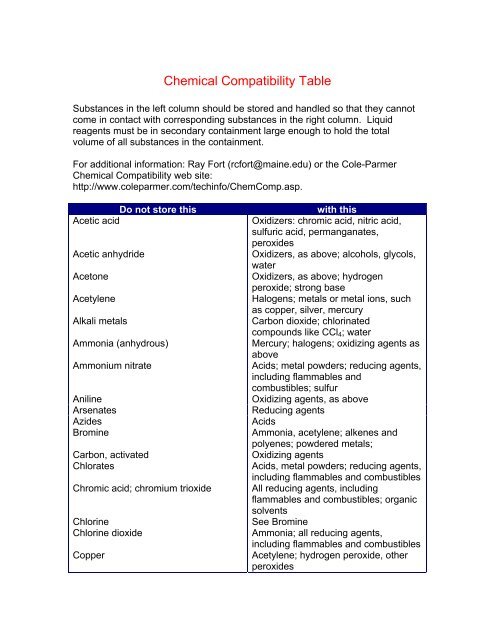
<strong>Chemical</strong> <strong>Compatibility</strong> <strong>Table</strong>Substances in the left column should be stored and handled so that they cannotcome in contact with corresponding substances in the right column. Liquidreagents must be in secondary containment large enough to hold the totalvolume of all substances in the containment.For additional information: Ray Fort (rcfort@maine.edu) or the Cole-Parmer<strong>Chemical</strong> <strong>Compatibility</strong> web site:http://www.coleparmer.com/techinfo/ChemComp.asp.Do not store thisAcetic acidAcetic anhydrideAcetoneAcetyleneAlkali metalsAmmonia (anhydrous)Ammonium nitrateAnilineArsenatesAzidesBromineCarbon, activatedChloratesChromic acid; chromium trioxideChlorineChlorine dioxideCopperwith thisOxidizers: chromic acid, nitric acid,sulfuric acid, permanganates,peroxidesOxidizers, as above; alcohols, glycols,waterOxidizers, as above; hydrogenperoxide; strong baseHalogens; metals or metal ions, suchas copper, silver, mercuryCarbon dioxide; chlorinatedcompounds like CCl 4 ; waterMercury; halogens; oxidizing agents asaboveAcids; metal powders; reducing agents,including flammables andcombustibles; sulfurOxidizing agents, as aboveReducing agentsAcidsAmmonia, acetylene; alkenes andpolyenes; powdered metals;Oxidizing agentsAcids, metal powders; reducing agents,including flammables and combustiblesAll reducing agents, includingflammables and combustibles; organicsolventsSee BromineAmmonia; all reducing agents,including flammables and combustiblesAcetylene; hydrogen peroxide, otherperoxides
CyanidesFlammable liquidsFluorineHydrazineHydrocarbons, flammableHydrofluoric acidHydrogen peroxideHydrogen sulfideHypochloritesIodineMercuryNitratesNitric acid (concentrated)NitritesNitroalkanesOxalic acidOxygenPerchloric acidPeroxides (organic)Phosphorus (white)PotassiumPotassium perchloratePotassium permanganateSelenidesSilver, silver saltsSodiumSodium peroxideSulfuric acidAcidsAll strong oxidizing agents, halogensStorage not permittedAll strong oxidizers, esp. hydrogenperoxideAll strong oxidizers; halogensStorage not permittedMetals and metal salts; flammablesand combustibles; aromatic amines;discard after three monthsAll strong oxidizersAcids, activated carbon; strongreducing agentsAcetylene; ammonia, anhydrous oraqueousAcetylene; nitric acid; ammoniaAcids; all reducing agents andflammablesAll organics; all flammables; chromicacid; hydrohalic acids; hydrogen sulfideAcids; strong oxidizers; strong reducingagentsStrong base; aminesSilver and silver salts; mercury andmercury salts; strong oxidizing agentsAll flammable and combustibleorganics, including oil and greaseAll organics; all metals except stainlesssteelAcids; heat; friction and shockAir; oxygenOrganic halides; water; alcohols ofthree carbons or fewer; CO 2See Perchloric acidGlycerol, other polyols; low MWaldehydes; sulfuric acidReducing agents (produce H 2 Se)Acetylene; dicarboxylic acids;ammonium saltsSee potassiumAll oxidizable organicsEasily oxidizable organics; hydrohalicacids; chlorates and perchlorates;permanganates