DOUBLE REPLACEMENT PRACTICE REACTIONS example
DOUBLE REPLACEMENT PRACTICE REACTIONS - Library
DOUBLE REPLACEMENT PRACTICE REACTIONS - Library
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
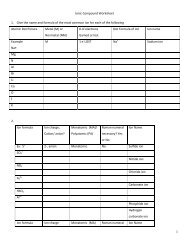
12) Ag 2 S + CuCl 2 --->13) Pb(NO 3 ) 2 + HI --->14) Ba(ClO 3 ) 2 + H 2 SO 4 --->15) CuS + KCl --->SINGLE <strong>REPLACEMENT</strong>REACTION DESCRIPTIONIn these reactions, a free element reacts with a compoundto form another compound and release one of the elementsof the original compound in the elemental state. There aretwo different possibilities:1. One cation (+ ion) replaces another.2. One anion (- ion) replaces another.REACTION FORMAT1. AX+ B ----> BX + A2. Y + AX ----> AY + XREACTION GUIDELINES1. In a single replacement reaction atoms of one element replacethe atoms of a second element in a compound.A metal will replace another metal from a compoundA nonmetal will replace another nonmetal from a compoundReplacement Worksheet-Chem 1213