Pre-Conference Tutorials, Monday, March 8, 2010, 09:00
Pre-Conference Tutorials, Monday, March 8, 2010, 09:00
Pre-Conference Tutorials, Monday, March 8, 2010, 09:00
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
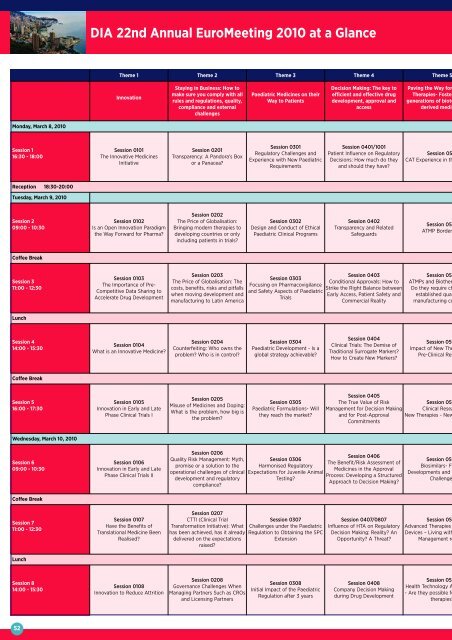
<strong>Monday</strong>, <strong>March</strong> 8, <strong>2010</strong><br />
Session 1<br />
16:30 - 18:<strong>00</strong><br />
Reception 18:30-20:<strong>00</strong><br />
Tuesday, <strong>March</strong> 9, <strong>2010</strong><br />
Session 2<br />
<strong>09</strong>:<strong>00</strong> - 10:30<br />
Coffee Break<br />
Session 3<br />
11:<strong>00</strong> - 12:30<br />
Lunch<br />
Session 4<br />
14:<strong>00</strong> - 15:30<br />
Coffee Break<br />
Session 5<br />
16:<strong>00</strong> - 17:30<br />
Wednesday, <strong>March</strong> 10, <strong>2010</strong><br />
Session 6<br />
<strong>09</strong>:<strong>00</strong> - 10:30<br />
Coffee Break<br />
Session 7<br />
11:<strong>00</strong> - 12:30<br />
Lunch<br />
Session 8<br />
14:<strong>00</strong> - 15:30<br />
52<br />
DIA 22nd Annual EuroMeeting <strong>2010</strong> at a Glance<br />
Theme 1 Theme 2 Theme 3 Theme 4 Theme 5<br />
Innovation<br />
Session 0101<br />
The Innovative Medicines<br />
Initiative<br />
Session 0102<br />
Is an Open Innovation Paradigm<br />
the Way Forward for Pharma?<br />
Session 0103<br />
The Importance of <strong>Pre</strong>-<br />
Competitive Data Sharing to<br />
Accelerate Drug Development<br />
Session 0104<br />
What is an Innovative Medicine?<br />
Session 0105<br />
Innovation in Early and Late<br />
Phase Clinical Trials I<br />
Session 0106<br />
Innovation in Early and Late<br />
Phase Clinical Trials II<br />
Session 0107<br />
Have the Benefits of<br />
Translational Medicine Been<br />
Realised?<br />
Session 0108<br />
Innovation to Reduce Attrition<br />
Staying in Business: How to<br />
make sure you comply with all<br />
rules and regulations, quality,<br />
compliance and external<br />
challenges<br />
Session 0201<br />
Transparency: A Pandora’s Box<br />
or a Panacea?<br />
Session 0202<br />
The Price of Globalisation:<br />
Bringing modern therapies to<br />
developing countries or only<br />
including patients in trials?<br />
Session 0203<br />
The Price of Globalisation: The<br />
costs, benefits, risks and pitfalls<br />
when moving development and<br />
manufacturing to Latin America<br />
Session 0204<br />
Counterfeiting: Who owns the<br />
problem? Who is in control?<br />
Session 0205<br />
Misuse of Medicines and Doping:<br />
What is the problem, how big is<br />
the problem?<br />
Session 0206<br />
Quality Risk Management: Myth,<br />
promise or a solution to the<br />
operational challenges of clinical<br />
development and regulatory<br />
compliance?<br />
Session 0207<br />
CTTI (Clinical Trial<br />
Transformation Initiative): What<br />
has been achieved, has it already<br />
delivered on the expectations<br />
raised?<br />
Session 0208<br />
Governance Challenges When<br />
Managing Partners Such as CROs<br />
and Licensing Partners<br />
Paediatric Medicines on their<br />
Way to Patients<br />
Session 0301<br />
Regulatory Challenges and<br />
Experience with New Paediatric<br />
Requirements<br />
Session 0302<br />
Design and Conduct of Ethical<br />
Paediatric Clinical Programs<br />
Session 0303<br />
Focusing on Pharmacovigilance<br />
and Safety Aspects of Paediatric<br />
Trials<br />
Session 0304<br />
Paediatric Development - Is a<br />
global strategy achievable?<br />
Session 0305<br />
Paediatric Formulations- Will<br />
they reach the market?<br />
Session 0306<br />
Harmonised Regulatory<br />
Expectations for Juvenile Animal<br />
Testing?<br />
Session 0307<br />
Challenges under the Paediatric<br />
Regulation to Obtaining the SPC<br />
Extension<br />
Session 0308<br />
Initial Impact of the Paediatric<br />
Regulation after 3 years<br />
Decision Making: The key to<br />
efficient and effective drug<br />
development, approval and<br />
access<br />
Session 0401/1<strong>00</strong>1<br />
Patient Influence on Regulatory<br />
Decisions: How much do they<br />
and should they have?<br />
Session 0402<br />
Transparency and Related<br />
Safeguards<br />
Session 0403<br />
Conditional Approvals: How to<br />
Strike the Right Balance between<br />
Early Access, Patient Safety and<br />
Commercial Reality<br />
Session 0404<br />
Clinical Trials: The Demise of<br />
Traditional Surrogate Markers?<br />
How to Create New Markers?<br />
Paving the Way for<br />
Therapies- Foster<br />
generations of biote<br />
derived medic<br />
Session 05<br />
CAT Experience in th<br />
Session 05<br />
ATMP Border<br />
Session 05<br />
ATMPs and Biother<br />
Do they require ch<br />
established qua<br />
manufacturing co<br />
Session 050<br />
Impact of New The<br />
<strong>Pre</strong>-Clinical Res<br />
Session 0405<br />
The True Value of Risk<br />
Session 05<br />
Management for Decision Making Clinical Resea<br />
and for Post-Approval New Therapies - New<br />
Commitments<br />
Session 0406<br />
The Benefit/Risk Assessment of<br />
Medicines in the Approval<br />
Process: Developing a Structured<br />
Approach to Decision Making?<br />
Session 0407/0807<br />
Influence of HTA on Regulatory<br />
Decision Making: Reality? An<br />
Opportunity? A Threat?<br />
Session 0408<br />
Company Decision Making<br />
during Drug Development<br />
Session 050<br />
Biosimilars- F<br />
Developments and<br />
Challenge<br />
Session 05<br />
Advanced Therapies<br />
Devices – Living with<br />
Management re<br />
Session 05<br />
Health Technology A<br />
- Are they possible fo<br />
therapies?