Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
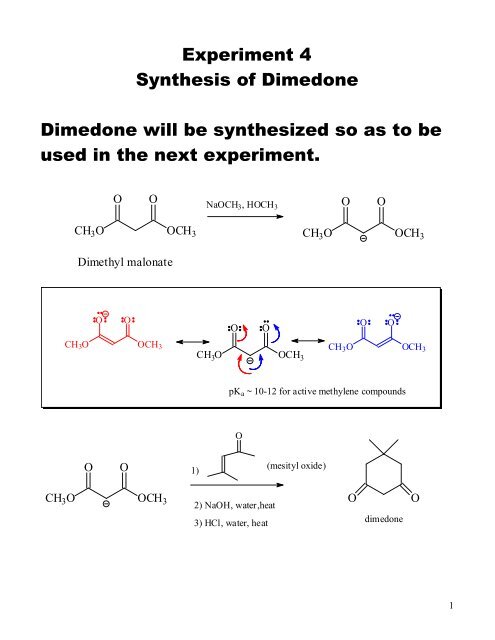
Experiment 4<br />
Synthesis of <strong>Dimedone</strong><br />
<strong>Dimedone</strong> will be synthesized so as to be<br />
used in the next experiment.<br />
O O<br />
CH 3O OCH 3<br />
Dimethyl malonate<br />
O O<br />
CH3O OCH3<br />
O O 1)<br />
CH 3O OCH 3<br />
NaOCH3, HOCH3<br />
O O<br />
CH 3O OCH 3<br />
O O<br />
CH 3O OCH 3<br />
O O<br />
CH3O OCH3<br />
pKa ~ 10-12 for active methylene compounds<br />
O<br />
2) NaOH, water,heat<br />
3) HCl, water, heat<br />
(mesityl oxide)<br />
O O<br />
dimedone<br />
1
Let’s look at the experiments we have<br />
done so far:<br />
Exp 1 (Cycloaddition) , Exp 2 (Nitration of<br />
Naphthalene) and Exp 3<br />
(Aldol/dehydration): Mix substances,<br />
isolate product, recrystallize<br />
Exp 4 (Synthesis of <strong>Dimedone</strong>):<br />
Mix substances, then hydrolyze, then<br />
decarboxylate. Eventually isolate product<br />
and recrystallize.<br />
More steps – more chances for lower<br />
product yield AND purity.<br />
Lower yield:<br />
�Glassware must be dry<br />
�If too much solvent is used in<br />
recrystallization step, lower yield<br />
Lower purity:<br />
�If recrystallized product not<br />
properly washed, lower purity<br />
2
Step 1:<br />
O O<br />
CH 3O OCH 3<br />
O<br />
H<br />
O<br />
H<br />
H<br />
H<br />
H<br />
H H<br />
O<br />
O OCH 3<br />
OCH 3<br />
Keto-enol tautomerism<br />
(see Vollhardt, p. 772)<br />
O<br />
H<br />
O OCH3 H<br />
OCH 3<br />
Na OCH3<br />
O O<br />
HOCH 3 CH 3O OCH 3<br />
The anion of dimethyl<br />
malonate adds in a 1,4addition.<br />
CH 3 O H<br />
OCH3<br />
CH3O H<br />
O<br />
O<br />
H<br />
O<br />
1<br />
3<br />
2<br />
4<br />
O<br />
O OCH 3<br />
O<br />
O OCH 3<br />
OCH 3<br />
OCH 3<br />
There are three sets of acidic protons.<br />
Let’s pull off one of the H , follow it<br />
through, and then explain why the other<br />
types of protons don’t lead to a product.<br />
3
pKa ~ 19 H<br />
H<br />
O<br />
H<br />
H<br />
O<br />
H<br />
O OCH3 H<br />
pKa ~ 19<br />
O<br />
O O<br />
OCH 3<br />
pKa ~10<br />
OCH3<br />
+ OCH 3<br />
Na OCH3<br />
O<br />
O<br />
3<br />
2<br />
1<br />
3 4<br />
2<br />
4<br />
5<br />
CH 3O<br />
1<br />
6<br />
5<br />
O<br />
O<br />
6<br />
O<br />
OCH 3<br />
OCH 3<br />
O<br />
OCH 3<br />
If you take off a H or a H proton and try to do the<br />
cyclization, you will produce a 4-membered ring<br />
(see Vollhardt, Chapter 23) – not allowed.<br />
O<br />
H<br />
H<br />
H<br />
H<br />
O<br />
H<br />
O<br />
H<br />
OCH 3<br />
OCH 3<br />
N a O CH 3<br />
O<br />
H<br />
1<br />
H<br />
H<br />
O<br />
1<br />
O<br />
2<br />
3<br />
H<br />
4<br />
O OCH3 H<br />
X<br />
2 3<br />
O<br />
4<br />
O<br />
OCH 3<br />
OCH3<br />
OCH 3<br />
not allowed<br />
4
Step 2:<br />
O<br />
O O<br />
O O<br />
OH<br />
O OH<br />
OCH 3<br />
OCH 3<br />
NaOH(aq)<br />
K = 1<br />
O O<br />
OCH3<br />
K=10 11<br />
O<br />
O O<br />
CO 2<br />
O H<br />
+ CH 3 OH<br />
"drives"<br />
hydrolysis<br />
pKa ~ 5<br />
Esters react with – OH, therefore must<br />
keep H 2O out of reaction of dimethyl<br />
malonate with mesityl oxide<br />
*** CH 3OH removed BEFORE acid<br />
hydrolysis (step 3) by rotary evaporator;<br />
equilibrium will favor carboxylate.<br />
Hydrolysis reaction temperature, for step<br />
3, will be higher without methanol<br />
present.<br />
***<br />
5
Step 3:<br />
O O<br />
CO 2<br />
H<br />
O O H<br />
H<br />
O O H<br />
H3O + (aq)<br />
pKa ~ -2<br />
K = 10 7<br />
H O H<br />
H<br />
acid catalyzed Enol-<br />
Keto Equilibration<br />
(see Vollhardt, p 772)<br />
H O H<br />
ΔG ~ -17 Kcal/mol; K = 10 17<br />
4<br />
O<br />
5<br />
O O<br />
6<br />
3<br />
O 2<br />
O O H<br />
an enol<br />
O O<br />
H H<br />
pKa ~ 5.25, therefore<br />
the final solution must<br />
be acidic<br />
H 1<br />
+ CO 2<br />
+ H 3 O +<br />
6
Equipment for top equipment drawer:<br />
1. 250 mL one neck, round bottomed 24/40 flask<br />
2. reflux condenser with 24/40 standard taper joints<br />
3. 3 – 10 mL graduated cylinders<br />
4. 1 – 25 mL graduated cylinder<br />
5. 2 – 50 mL graduated cylinders<br />
6. 125 mL Erlenmeyer flask (to recrystallize crude dimedone)<br />
7. 250 mL filter flask<br />
8. small Büchner funnel<br />
9. Large black filter adapter (#3) for 250mL filter flask.<br />
10.2 – 200 mm double blade, stainless steel spatulas<br />
11.cork ring<br />
12.plastic stoppers for 24/40 standard taper joint<br />
13.drying tube<br />
14.one watch glass<br />
15.one stirring rod<br />
PROCEDURE:
Useful data: Sodium methoxide, FW 54.02<br />
25 wt% NaOCH3 in methanol; d 0.945 g/mL<br />
Dimethyl malonate, FW 132.12; d 1.156 g/mL<br />
Mesityl oxide, FW 98.15; d 0.858 g/mL<br />
Minimize exposure of methanol or the reaction in progress to the atmosphere since<br />
these absorb moisture. You must get to the point of adding the 6 M HCl to the reaction<br />
before you leave lab the first day. If not, you will have to repeat the experiment.<br />
Place 12 mL of 25 wt% sodium methoxide solution in methanol in a 250 mL round<br />
bottomed flask equipped with a 24/40 standard taper joint. Add 5.7 mL of dimethyl<br />
malonate to the flask followed by a boiling stone. A small amount of solid may form<br />
when the dimethyl malonate is added (What is it likely to be?). Lightly grease the male<br />
24/40 standard taper joint of a reflux condenser and attach the condenser to the flask.<br />
(Note: It is important to lightly grease a standard taper joint that will be heated in the<br />
presence of base or stored for an extended period. Otherwise, the joint is likely to seize.)<br />
Attach a drying tube containing calcium chloride (aka Drierite) to the reflux condenser.<br />
Heat the reaction mixture to reflux using a sand bath. After 5 min at reflux, remove the<br />
reaction flask from the sand bath and let it cool for about 5 min. Briefly remove the drying<br />
tube from the reflux condenser and, with stirring, add 6.0 mL of mesityl oxide in several
portions into the flask through the condenser over ca. 2 min. Then reattach the drying tube<br />
and reheat the reaction mixture to reflux for 30 minutes. Cool the reaction flask to room<br />
temperature and remove the reflux condenser. Attach the reaction flask to a rotary<br />
evaporator and remove the methanol by rotary evaporation, while heating the flask with a<br />
warm water bath. When the standing liquid has been evaporated, remove the flask from<br />
the rotary evaporator. (The solid remaining in the flask will still be moist at this point.)<br />
Add 40 ml of 3 M aqueous NaOH solution to the reaction flask, attach the reflux<br />
condenser, and reheat the reaction mixture to reflux for 40 min using the sand bath.<br />
Remove the flask from the sand bath.<br />
Add 35 mL of a 6 M aqueous HCl solution to a 250 mL Erlenmeyer flask containing<br />
a boiling chip. While manually swirling the Erlenmeyer flask, carefully pour the warm<br />
reaction mixture into the flask. Check to see that the pH of the solution is about 1. If not,<br />
add more HCl solution in small portions and recheck the pH. Place a small watch glass<br />
over the mouth of the Erlenmeyer flask and boil the mixture gently, with stirring, for ca.<br />
10 min., until no more gas is evolving. At this stage the reaction can be left until the next<br />
lab period. If you choose to do this, loosely stopper the flask with a cork and carefully<br />
place the flask in YOUR STUDENT drawer. You must get a 250mL Erlenmeyer flask<br />
from the TA to put in your equipment drawer TO REPLACE the flask you were using this
session; otherwise you will be penalized. If you have time, however, collect the crude<br />
dimedone product as described below.<br />
MAKE SURE YOU HAVE A REAGENT TABLE WITH YOUR PLAN WHEN YOU<br />
COME TO LAB.