Terugbetalingssystemen voor geneesmiddelen ... - Pharma
Terugbetalingssystemen voor geneesmiddelen ... - Pharma
Terugbetalingssystemen voor geneesmiddelen ... - Pharma
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
8 Drug Reimbursement Systems KCE Reports 147<br />
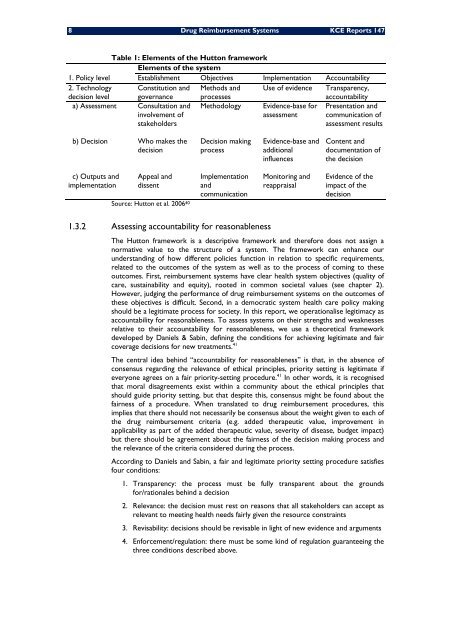
Table 1: Elements of the Hutton framework<br />
Elements of the system<br />
1. Policy level Establishment Objectives Implementation Accountability<br />
2. Technology Constitution and Methods and Use of evidence Transparency,<br />
decision level governance<br />
a) Assessment Consultation and<br />
involvement of<br />
stakeholders<br />
processes<br />
Methodology<br />
Evidence-base for<br />
assessment<br />
accountability<br />
Presentation and<br />
communication of<br />
assessment results<br />
b) Decision Who makes the<br />
decision<br />
Decision making<br />
process<br />
Evidence-base and<br />
additional<br />
influences<br />
Content and<br />
documentation of<br />
the decision<br />
c) Outputs and<br />
implementation<br />
Appeal and<br />
dissent<br />
Source: Hutton et al. 2006 40<br />
Implementation<br />
and<br />
communication<br />
Monitoring and<br />
reappraisal<br />
Evidence of the<br />
impact of the<br />
decision<br />
1.3.2 Assessing accountability for reasonableness<br />
The Hutton framework is a descriptive framework and therefore does not assign a<br />
normative value to the structure of a system. The framework can enhance our<br />
understanding of how different policies function in relation to specific requirements,<br />
related to the outcomes of the system as well as to the process of coming to these<br />
outcomes. First, reimbursement systems have clear health system objectives (quality of<br />
care, sustainability and equity), rooted in common societal values (see chapter 2).<br />
However, judging the performance of drug reimbursement systems on the outcomes of<br />
these objectives is difficult. Second, in a democratic system health care policy making<br />
should be a legitimate process for society. In this report, we operationalise legitimacy as<br />
accountability for reasonableness. To assess systems on their strengths and weaknesses<br />
relative to their accountability for reasonableness, we use a theoretical framework<br />
developed by Daniels & Sabin, defining the conditions for achieving legitimate and fair<br />
coverage decisions for new treatments. 41<br />
The central idea behind “accountability for reasonableness” is that, in the absence of<br />
consensus regarding the relevance of ethical principles, priority setting is legitimate if<br />
everyone agrees on a fair priority-setting procedure. 41 In other words, it is recognised<br />
that moral disagreements exist within a community about the ethical principles that<br />
should guide priority setting, but that despite this, consensus might be found about the<br />
fairness of a procedure. When translated to drug reimbursement procedures, this<br />
implies that there should not necessarily be consensus about the weight given to each of<br />
the drug reimbursement criteria (e.g. added therapeutic value, improvement in<br />
applicability as part of the added therapeutic value, severity of disease, budget impact)<br />
but there should be agreement about the fairness of the decision making process and<br />
the relevance of the criteria considered during the process.<br />
According to Daniels and Sabin, a fair and legitimate priority setting procedure satisfies<br />
four conditions:<br />
1. Transparency: the process must be fully transparent about the grounds<br />
for/rationales behind a decision<br />
2. Relevance: the decision must rest on reasons that all stakeholders can accept as<br />
relevant to meeting health needs fairly given the resource constraints<br />
3. Revisability: decisions should be revisable in light of new evidence and arguments<br />
4. Enforcement/regulation: there must be some kind of regulation guaranteeing the<br />
three conditions described above.