Instrument Care
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
1 <strong>Instrument</strong> <strong>Care</strong><br />
<strong>Instrument</strong><br />
<strong>Care</strong><br />
The content is of prime importance !!!<br />
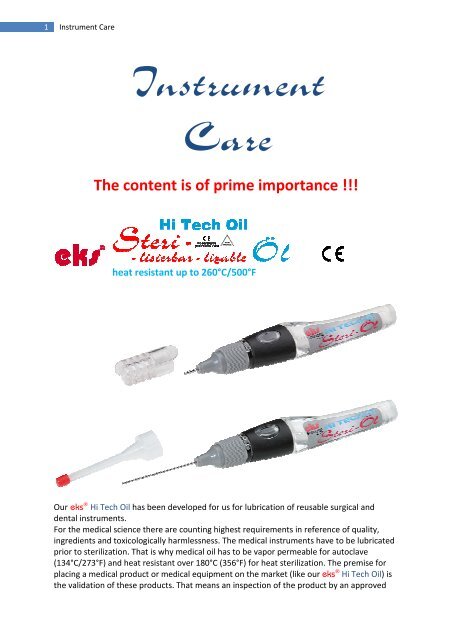
heat resistant up to 260°C/500°F<br />
Our eks ® Hi Tech Oil has been developed for us for lubrication of reusable surgical and<br />
dental instruments.<br />
For the medical science there are counting highest requirements in reference of quality,<br />
ingredients and toxicologically harmlessness. The medical instruments have to be lubricated<br />
prior to sterilization. That is why medical oil has to be vapor permeable for autoclave<br />
(134°C/273°F) and heat resistant over 180°C (356°F) for heat sterilization. The premise for<br />
placing a medical product or medical equipment on the market (like our eks ® Hi Tech Oil) is<br />
the validation of these products. That means an inspection of the product by an approved
2 <strong>Instrument</strong> <strong>Care</strong><br />
laboratory which proves the conformity to the European guideline 93/42/EEC for medical<br />
devices to achieve the admission as medical product. We have assigned the well-respected<br />
laboratory SGS Institut Fresenius to verify the eks ® Hi Tech Oil that we are allowed to<br />
register this product as medical product according to 93/42/EEC and to achieve the<br />
admission for a medical product with the CE-marking.<br />
The most available instrument oils are not appropriate for heat sterilization. Our oil is<br />
approved for autoclave and heat sterilization.<br />
Furthermore our eks ® Hi Tech Oil is physiologically harmless, odorless, vapor permeable and<br />
equates the requirements of DAB and LFBG as well as USDA and FDA (H1) and is heat<br />
resistant up to 260°C (500°F) and leaves no encrustations/residues on the surfaces.<br />
With this high quality standard the eks ® Hi Tech Oil is also applicable for the lubrication of:<br />
• Orthodontic and dental instruments<br />
• Veterinary instruments<br />
• Laboratory instruments<br />
• Chiropody-/podology instruments<br />
• Cosmetic and manicure instruments<br />
• Machines in food industry (food grade lubricant)<br />
Our eks ® Hi Tech Oil provides an excellent corrosion and oxidation protection and is<br />
odorless, transparent and free of silicone.<br />
For an order quantity of 300 pcs. private label is also available.
3 <strong>Instrument</strong> <strong>Care</strong><br />
Space for your private label<br />
Proposal<br />
Not to mention that the following documents are available on request:<br />
• MSDS<br />
• Product specification sheet<br />
• EC Declaration of Conformity (93/42/EEC)<br />
Dropwise eks ® -Quality Oil<br />
Precision Oiler<br />
ek 11-3 medic (7 mm tip stainless steel /<br />
inner ø 0,8 mm)<br />
℮ 12 ml / 0.4 fl.oz. / approx. 1000 drops<br />
ek 11-4 medic (40 mm tip stainless steel /<br />
inner ø 0,4 mm)<br />
℮ 12 ml / 0.4 fl.oz. / approx. 1100 drops<br />
ek 11-312 medic<br />
12 pcs. ek 11-3 medic (7 mm)<br />
Displays a 12 Oil-Pens<br />
ek 11-412 medic<br />
12 pcs. ek 11-4 medic (40 mm)<br />
ek 11-3.100 medic<br />
℮ 100 ml / 3.4 fl.oz.<br />
Fingertip Sprayer: no CFC
4 <strong>Instrument</strong> <strong>Care</strong><br />
®<br />
Measurements: 50 x 50 x 15 mm / 1.97” x 1.97” x 0.59”<br />
contains diamonds in 20-30 µm (Micron)<br />
for an excellent grinding result on satin finished surfaces<br />
Art.-No. ek 11-50<br />
A world first: We proudly present our brand new product, the eks ®<br />
® which is registered as medical product according to the<br />
European guideline 93/42/EEC, for reconditioning of reusable instruments with satin<br />
finished surfaces and for removal of kondensides, stains and corrosion. Our world first is that<br />
we combined our eks Diamond Rubbel with monocrystalline diamonds in 20-30 µm (Micron).<br />
The diamonds as the hardest known material is able to treat any surface. Also handy for<br />
gently cleaning porcelain or just about any ceramic surface. As a matter of course the eks ®<br />
®<br />
is a product Made in Germany.<br />
The eks ®<br />
® is also the perfect tool for cleaning surgical and<br />
dental burs, sterilization container, stainless steel tables and operating rooms. It removes<br />
the fine metallic residue keeping the rod in like new condition. Over time the buildup will<br />
make the burs super smooth and it just won't do much. This will help restore its<br />
abrasiveness.<br />
eks ® ® for care of satin finished stainless steel surfaces
5 <strong>Instrument</strong> <strong>Care</strong><br />
For satin finished<br />
surfaces<br />
size of pads: 12,5 x 10,0 cm / 4.92” x 3.94”<br />
black pad: grain 180 (Art.-No. ek 11-62)<br />
red pad: grain 320 (Art.-No. ek 11-64)<br />
grey pad: grain 600 (Art.-No. ek 11-66)<br />
Set of three assorted Satin Pads<br />
(grain 180, 320 and 600)<br />
(Art.-No. ek 11-60)<br />
The eks ®<br />
contain high performance fibers with flexible resin technology.<br />
Combined with a high mineral content the eks ®<br />
possess flexibility and<br />
adaptiveness which is essential to achieve a plain surface on each workpiece.<br />
All well-respected Manufacturers of reusable surgical instruments are already using this<br />
material to satin finish their instruments during the manufacturing process.<br />
We have made this material into handy size for reconditioning of surgical instruments. Even<br />
the serrated edges are ideal for delicate conditioning of detents and box/lap joints.
6 <strong>Instrument</strong> <strong>Care</strong><br />
double sided 12,000 MM grit for polishing<br />
(14 x 14 cm / 5.51” x 5.51”)<br />
For high polished<br />
surfaces<br />
Art.-No. ek 11-70<br />
This micro polishing linen with a grit of 12,000 MM is ideal for polishing mirror polished<br />
stainless steel instruments. Also for polishing wood, plastic, acrylic glass, glass fibre, high<br />
gloss paint and porcelain. The grinding crystals are bonded in a silicone matrix on a thin and<br />
flexible mesh. So, no residues will be left on the processed surface. Medic Mesh can be used<br />
dry or with water and has a high durability at normal strain.<br />
The eks ®<br />
is double sided and is as well registered as medical<br />
product according to the European guideline for medical products 93/42/EEC. /EEC.
7 <strong>Instrument</strong> <strong>Care</strong><br />
including Hi Tech Oil ek 11-3 medic<br />
Medical product according to 93/42/EEC for surgical instruments as well easy to use for all<br />
other fine surfaces.<br />
Art.-No. ek 11-300 medic - Cleaning Set<br />
Set contains:<br />
• 3 pcs. single sided Medic Mesh polishing linen 7,5 x 10 cm / 2.95” x 3.49” with three<br />
different grains: 2400, 4000 (for preliminary work) and 12000 MM (for mirror<br />
polishing)<br />
• 1 box<br />
• 1 foam block for using with medic mesh<br />
• 1 angle brush with nylon bristles<br />
• 1 surface brush with nylon bristles<br />
• 1 Oil Pen (7 mm stainless steel tip)
8 <strong>Instrument</strong> <strong>Care</strong><br />
<strong>Instrument</strong>s and burs cleaning brushes with nylon, brass, stainless steel bristles with<br />
plastic handle for single/daily use<br />
ek 11-80<br />
ek 11-82<br />
ek 11-84<br />
ek 11-86<br />
ek 11-5<br />
ek 11-5.1<br />
ek 4-72 p<br />
brushes with stainless steel bristles<br />
brushes with brass bristles<br />
brushes with nylon bristles (autoclavable)<br />
brushes with double sided<br />
nylon bristles (autoclavable)<br />
brush with brass bristles<br />
brush with nylon bristles<br />
brush with nylon bristles and cleaning rubber<br />
Please consider a careful use of brass and stainless brushes. These can harm<br />
delicate surfaces!
9 <strong>Instrument</strong> <strong>Care</strong><br />
Scalpel Ex<br />
For a safe removal of scalpel blades<br />
Made by eks-Solingen Germany<br />
ek 10-1 r<br />
Scalpel blade remover, stainless steel<br />
Simply to use: 1. Insert the blade into the tool and 2. press the tool together towards the<br />
blade. This releases the blade from the scalpel handle and 3. holds the blade in the tool<br />
while removing.
10 <strong>Instrument</strong> <strong>Care</strong><br />
Steri-Forceps<br />
ek 1-125125 r Steri-Forceps 25 cm, stainless steel<br />
ek 1-130130 r Steri-Forceps 30 cm, stainless steel<br />
ek 1-100 r<br />
Tweezers for taking hold of instruments and burs,<br />
stainless steel
11 <strong>Instrument</strong> <strong>Care</strong><br />
eks eduard kühnert GmbH<br />
Mangenberger Strasse 264<br />
42655 Solingen<br />
Tel. +49 (0) 212 338289<br />
Fax: +49 (0) 212 329970<br />
eks@eks-solingen.de<br />
www.eks-solingen.de<br />
Directions for preparation and use<br />
Reusable instruments Class I<br />
Part I:<br />
Part II:<br />
General remarks<br />
Remarks on preparation<br />
1. Scope<br />
14. General principles<br />
2. Principles<br />
in relation to hygiene and preparation<br />
3. Appropriate usage 15. Preparation for cleaning<br />
4. Restrictions and disinfection<br />
5. Warning notes 16. Manual cleaning and disinfection<br />
6. Labelling, symbols on<br />
labels<br />
17. Ultrasonic cleaning<br />
7. Combination with other 18. Machine cleaning –<br />
products<br />
thermal disinfection<br />
8. Materials<br />
19. Control and maintenance<br />
9. Stability of materials 20. Packaging<br />
10. Disposal; return 21. Sterilization<br />
11. Warranty<br />
22. Storage<br />
12. Manufacturer – Service contact 23. Confirmation – Note<br />
13. Standards – References<br />
Part I: General remarks<br />
1. Scope<br />
All reusable surgical instruments that<br />
• consist of a single part<br />
• contain simple articulations or<br />
• or easily moved parts<br />
• including those made from a combination of several replaceable parts (e.g. a handle component and a number of different specialized<br />
inserts).<br />
Not included are products that are<br />
⊗ connected up to an powered device<br />
⊗ themselves powered externally<br />
⊗ made entirely of non-metallic materials.<br />
2. Principles<br />
These instructions for use are not a substitute for training, due care and knowledge of current technology on the part of the user. For this reason we<br />
assume that users have training in and knowledge of the applicable legislation, regulations and recommendations (from e.g. the RKI 2 and the AKI 1 --<br />
see "Regulations – References) and therefore restrict ourselves to providing the instructions and information relating to our products that the user<br />
must observe when handling individual instruments. The reasoning behind these instructions and the hazards arising from non-observance of them<br />
are listed in the relevant legislation and recommendations.<br />
PLEASE READ THESE INSTRUCTIONS FOR USE VERY CAREFULLY BEFORE PREPARING THE PRODUCT FOR THE FIRST TIME AND USING IT<br />
3. Appropriate usage<br />
These instruments should only be use for their intended purpose in the relevant medical specializations by appropriately trained and qualified<br />
personnel.<br />
The person responsible for the selection of this equipment for particular applications and for operational usage, for the appropriate training and for<br />
possession of information and the sufficient experience for handling the equipment shall be the attending physician and/or user.<br />
4. Restrictions<br />
Frequent repeated preparation will have only a minor effect on the equipment's service life, which is determined by the level of wear, damage and<br />
misuse of the equipment.<br />
After use on patients with Creutzfeldt-Jacob's disease (CJD) or on any variant of that complaint, we do not accept any responsibility for any<br />
consequences of its reuse.
12 <strong>Instrument</strong> <strong>Care</strong><br />
We recommend destroying the instruments after such use. Also, any preparation and reuse in accordance with the RKI 2 guideline shall be entirely at<br />
the user's own responsibility!<br />
<strong>Instrument</strong>s containing aluminium will be damaged by the use of alkaline cleaners > pH 7!<br />
5. Warning notes<br />
<strong>Instrument</strong>s are generally delivered in NON-STERILE condition!<br />
After receiving the products, check the delivery for correctness, completeness, integrity and correct functioning before subjecting the instruments to<br />
preparation.<br />
Before any use of the instruments, they should be checked for breakages, cracks, deformation, damage and readiness for functioning. Check for such<br />
issues as cutting, sharpening, closing, locking, denting and the correct functioning of all moving parts. Worn, corroded, deformed, porose or<br />
otherwise damaged instruments should be removed.<br />
6. Labelling – Symbols on labels<br />
7. Combinations with other products<br />
If instruments are reassembled after being dismantled, particular individual parts should not be replaced using parts from another manufacturer.<br />
If the designated purpose of the product allows it to be replaced (e.g. various work inserts), no components produced by another manufacturer may<br />
be used to replace it!<br />
We recommend that even other accessories (e.g. eks ® sterilizable Oil, eks ® Cleaning and <strong>Care</strong> Set, eks ® Satin Pads, eks ® Diamond Rubbel and eks ®<br />
<strong>Instrument</strong> Cleaning Brushes) should be sourced from eks eduard kühnert GmbH.<br />
8. Materials<br />
Steels conforming to DIN EN ISO 7153-11 for medical instruments.<br />
Plastics authorized for medical products and tested for bio-compatibility.<br />
9. Stability of materials<br />
Cleaning and disinfection agents should not contain the following ingredients:<br />
• organic, mineral and oxidising acids<br />
• strong basic materials (> pH 11, mildly alkaline cleaning agents recommended),<br />
• halogenated hydrocarbons, chlorine, iodine,<br />
• organic solvents (alcohols, acetone, etc.).<br />
• ammonia<br />
These products are thermally stable and may be exposed to temperatures of up to a maximum of 180°C.<br />
10. Disposal; Return<br />
Returns to eks eduard kühnert GmbH are only accepted if they have been declared hygienically safe" (treated by a disinfection process) or<br />
labelled "not decontaminated", securely packed and sterilized.<br />
After being disinfected successfully, defective or obsolete instruments<br />
should be disposed of appropriately or delivered to a suitable recycling system.<br />
11. Warranty<br />
Safety note: Appropriate cleaning, disinfection and sterilization is the responsibility of the operator/product user. National regulations, including any<br />
restrictions to these instructions, must be strictly observed<br />
eks eduard kühnert GmbH only supplies fully tested and entirely flaw-free free products to its customers. All our products are designed and<br />
manufactured to conform to the highest possible quality requirements.<br />
eks eduard kühnert GmbH, as manufacturer of these products, excludes all l warranty claims and does not accept any responsibility for<br />
direct or consequent damages that have been caused by the following:<br />
• usage for a purpose other than that for which they were designed<br />
• improper usage, application or handling<br />
• improper preparation and sterilization<br />
• improper maintenance and repair<br />
• non-observance observance of these instructions for use<br />
Repairs should be carried out only by organisations and individuals authorized by eks eduard kühnert. . Doing otherwise will lead to the<br />
exclusion of any warranty claims.
13 <strong>Instrument</strong> <strong>Care</strong><br />
12. Manufacturer – Service contact<br />
If any uncertainties, disagreements or questions should arise, please contact us before preparing, using or re-using the product.<br />
eks eduard kühnert GmbH<br />
Mangenberger Strasse – D-42655 Solingen<br />
Tel. +49 (0) 212 / 338289<br />
Fax: +49 (0) 212 / 329970<br />
Mail: eks@eks-solingen.de<br />
13. Standards – References<br />
• AKI 1 - Guidelines "Preparing instruments correctly"<br />
• RKI 2 - Recommendation: "Hygiene requirements when preparing medical devices"<br />
• DIN EN 285 Large steam sterilizers<br />
• DIN EN 13060 Small steam sterilizers<br />
• DIN EN ISO 15883-1-3 Cleaning and disinfection devices<br />
• DIN EN 868/ ANSI AAMI ISO 11607 packaging materials<br />
• DIN EN ISO 17664 Sterilization - information from manufacturer<br />
• DIN EN ISO 17665-1 (and/or DIN EN 554)/ ANSI AAMI ISO 11134<br />
Sterilization process – Moist heat<br />
1 AKI: Arbeitskreis <strong>Instrument</strong>en-Aufbereitung (Working group on instrument preparation)<br />
2 RKI: Robert-Koch-Institut<br />
Part II: Remarks on preparation<br />
14. General principles on hygiene and preparation<br />
• Brand new instruments and instrument sent back after repair should be prepared for first time usage as if they had already been used.<br />
The protective packaging for transport, protective caps, etc, are not suitable for sterilisation.<br />
• Use only authorised agents (RKI, DGHM/ VHA, FDA, etc.).<br />
• Alkaline and pH-neutral cleaning agents may be used.<br />
• CAUTION: DO NOT USE ANY ALKALINE CLEANERS >PH/ ON INSTRUMENTS CONTAINING ALUMINIUM<br />
• Water quality conforming to DIN EN 285 Appendix B.<br />
• Sterilizers conforming to DIN EN 285 or DIN EN 13060.<br />
• Cleaning and disinfection devices conforming to DIN EN ISO 15883 Parts 1 and 2.<br />
• Use only procedures sufficiently validated for the particular device and product for cleaning/disinfection/sterilization.<br />
• Strictly observe all manufacturer's instructions and recommendations.<br />
• In addition, observe all legislative provisions and hygiene regulations in force in your country. This applies in particular to the various<br />
rules in relation to effective deactivation of prions.<br />
15. Preparation at the place of use and for cleaning/disinfection<br />
• Remove residues from the application immediately!<br />
• Do not use any metal brushes or steel wool!<br />
• Do NOT soak in salt (NaCl) solution!<br />
• Never leave instruments under stress loads, leave jointed instruments in open position,<br />
• Disassemble dismountable instruments, subject narrow-necked instruments and parts to special pretreatment!<br />
• Handle and store all instruments appropriately!<br />
• Wet disposal: Wait max. 1 hour before preparation!<br />
• Dry disposal: Wait max. 3 hours before preparation!<br />
16. Manual cleaning and disinfection<br />
• Machine cleaning/disinfection is always preferable to manual cleaning!<br />
• Manual cleaning should only be used where no machine is available and in exceptional cases. Even in such situations, additional<br />
validation processes appropriate to the product and process will be required and will be the responsibility of the user.<br />
• Do not use any metal brushes or steel wool!<br />
• Clean narrow necked instruments and parts with particular care! Page 2 of 3<br />
• Handle and store all instruments appropriately!<br />
17. Ultrasonic cleaning<br />
• Maximum temperature: 50 ° C.<br />
• Frequency: 35 – 45 kHz<br />
• Required cleaning time: 4-5 minutes<br />
• Insert jointed instruments in open position!<br />
• Fill instruments containing lumina with fluid ensuring that no trapped bubbles are present, aligning them appropriately for the ultrasonic<br />
effect!<br />
18. Machine cleaning – thermal disinfection<br />
• Machine cleaning/thermal disinfection is the preferable method to be used!<br />
• Never leave instruments under stress loads, leave jointed instruments in open position, disassemble dismountable instruments, position<br />
narrow-necked instruments and parts with special care and/or use specialised rinsing equipment!<br />
• Handle and store all instruments appropriately!<br />
• Temperature for disinfection max. 95°C<br />
• A0 –Value (duration/temperature) in accordance with the rating of the product according to the RKI 2 - guideline!
14 <strong>Instrument</strong> <strong>Care</strong><br />
19. Control and maintenance<br />
• <strong>Instrument</strong>s must be allowed cool to room temperature!<br />
• Reassemble instruments to check correct functioning!<br />
• Joints, threads and slide faces should be treated with, e.g. eks ® sterilizable Oil after cleaning/disinfection, but before checking for correct<br />
functioning and sterilization. Only use other treatments (paraffin or white oil based and silicon-free agents) only if they are approved and<br />
validated for steam and hot-air sterilization and have been checked for bio-compatibility.<br />
• Pick out damaged instruments, check that they have been cleaned/disinfected properly (and repeat if necessary), and send them back to<br />
us.<br />
20. Packing<br />
• No special requirements.<br />
• Packaging conforming to DIN EN 868/ ANSI AAMI ISO 11607 may be used.<br />
21. Sterilization<br />
• Heat and steam sterilization is permitted!<br />
• Other sterilization procedures and the flash sterilization procedure are not permitted.<br />
• The fractionated vacuum procedure (with sufficient drying of product, of at least 15 min).<br />
Autoclave/Steam sterilization:<br />
• Maximum sterilization temperature 138 °C (280 °F, plus tolerance in accordance with DIN EN ISO 17665-1 (and/or DIN EN 554)/ ANSI<br />
AAMI ISO 11134.<br />
• Sterilization time (Exposure time to sterilization temperature) min. 20 min (at 121 °C (250 °F) or 5 min at 132 °C (270°F)/134 °C.<br />
• Steam sterilizer conforming to DIN EN 13060 or DIN EN 285<br />
• validated as conforming to DIN EN ISO 17665-1 (or DIN EN 554)/ ANSI AAMI ISO 11134 (valid commissioning and product-specific<br />
performance assessment).<br />
Hot-air sterilization:<br />
• While hot-air sterilization in accordance with AKI 1 (red brochure, section 10.2) no longer represents the latest state of science, the<br />
procedure is still used in specific cases: As long as a hot-air sterilizer remains in operation, the following special observations apply:<br />
• At temperatures above 185°C paraffin oil resinifies and thus no longer provides a lubrication function, thus reducing the functional<br />
performance of affected instruments. A synthetic oil such as eks ® sterilizable Oil has also been validated for hot-air sterilization and does<br />
not resinify at high temperatures, and thus does not take on any brown discolouration.<br />
• Where the intended temperature is substantially exceeded, there is a danger that of a loss of hardness and thus of functional<br />
performance as well as a danger of corrosion. This may cause many instruments to lose their practical functional value. In addition,<br />
plastics (e.g. coloured rings on instruments) may be damaged or destroyed by high temperatures.<br />
• In order to guarantee an even distribution of heat in the sterilization chamber and thus in the objects being sterilized, the indication on<br />
load volumes contained in the instructions for usage of the sterilizer's manufacturer must be strictly observed.<br />
• MIC instruments and endoscopes should never under any circumstance be sterilized using hot air.<br />
STERILIZATION IS NO SUBSTITUTE FOR CLEANLINESS<br />
22. Storage<br />
• Dry, protected from dust, protected from exterior forces, not subject to large fluctuations in temperature and not in the immediate<br />
vicinity of aggressive agents.<br />
• Appropriately on trays, in containers and cabinets.<br />
• There are otherwise no particular additional requirements.<br />
23. Confirmation – Note<br />
The above instructions have been judged appropriate for the preparation<br />
of medical instruments intended for reuse.<br />
It is the responsibility of the user (person in charge of preparation) to ensure the preparation actually carried out, with the equipment, material and<br />
personnel employed in the preparation facility achieves the required results. For this reason, validation and monitoring of the procedure's routines<br />
are normally necessary.<br />
In addition, every deviation that the user (person in charge of preparation) may make from the instructions prepared by each relevant manufacture<br />
should be carefully assessed for its individual effectiveness and for any possible negative effects of such deviations.
15 <strong>Instrument</strong> <strong>Care</strong><br />
eks eduard kühnert GmbH<br />
Mangenberger Str. 264<br />
42655 Solingen<br />
Germany<br />
Tel. +49 (0) 212 338289<br />
Fax. +49 (0) 212 653634<br />
eks@eks-solingen.de<br />
www.eks-solingen.de<br />
11/2015