WOW February 2017
Granisetron Transdermal System
Granisetron Transdermal System
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Writing on the Wall<br />
The Johns Hopkins Hospital Department of Pharmacy Newsletter <strong>February</strong> <strong>2017</strong> Vol. 17 Issue 2<br />
Inside this Issue<br />
A Summary of the<br />
<strong>2017</strong> GOLD Guidelines<br />
Update<br />
Angiotensin-<br />
Converting Enzyme<br />
Inhibitor and<br />
Angiotensin Receptor<br />
Blocker: Cost Saving<br />
Projections<br />
Granisetron<br />
Transdermal System 3<br />
NINJAs in the<br />
Children’s Center<br />
A Centralized<br />
Electronic Health<br />
Record: How Close<br />
Are We?<br />
Our “Epic” Summer 5<br />
Fundación Santa Fe de<br />
Bogotá<br />
1<br />
3<br />
4<br />
5<br />
7<br />
A Summary of the <strong>2017</strong> GOLD Guidelines Update<br />
Jessica Merrey, PharmD, MBA, BCPS, BCACP, CGP,<br />
Clinical Pharmacy Specialist, Ambulatory Care<br />
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) released an updated strategy for<br />
the diagnosis, management, and prevention of chronic obstructive lung disease (COPD) in November<br />
2016. This document was originally published in 2001, first updated in 2011, and has been updated annually<br />
since then. These updates have been fairly minor in terms of diagnosis and treatment until this year.<br />
The key changes in the <strong>2017</strong> GOLD guidelines can be segmented into three categories: refined ABCD<br />
assessment tool to further stratify risk, updated treatment algorithm for stable COPD and emphasis on<br />
risk of future exacerbations, and addition of hospital discharge and follow-up criteria, including use of<br />
integrated care team<br />
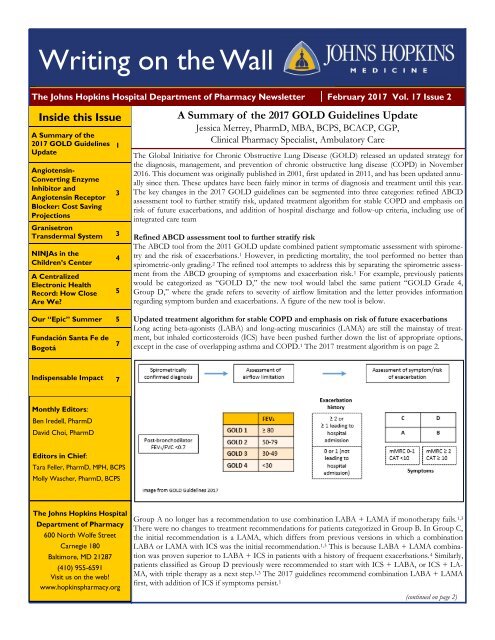
Refined ABCD assessment tool to further stratify risk<br />
The ABCD tool from the 2011 GOLD update combined patient symptomatic assessment with spirometry<br />
and the risk of exacerbations. 1 However, in predicting mortality, the tool performed no better than<br />
spirometric-only grading. 2 The refined tool attempts to address this by separating the spirometric assessment<br />
from the ABCD grouping of symptoms and exacerbation risk. 1 For example, previously patients<br />
would be categorized as “GOLD D,” the new tool would label the same patient “GOLD Grade 4,<br />
Group D,” where the grade refers to severity of airflow limitation and the letter provides information<br />
regarding symptom burden and exacerbations. A figure of the new tool is below.<br />
Updated treatment algorithm for stable COPD and emphasis on risk of future exacerbations<br />
Long acting beta-agonists (LABA) and long-acting muscarinics (LAMA) are still the mainstay of treatment,<br />
but inhaled corticosteroids (ICS) have been pushed further down the list of appropriate options,<br />
except in the case of overlapping asthma and COPD. 1 The <strong>2017</strong> treatment algorithm is on page 2.<br />
Indispensable Impact 7<br />
Monthly Editors:<br />
Ben Iredell, PharmD<br />
David Choi, PharmD<br />
Editors in Chief:<br />
Tara Feller, PharmD, MPH, BCPS<br />
Molly Wascher, PharmD, BCPS<br />
The Johns Hopkins Hospital<br />
Department of Pharmacy<br />
600 North Wolfe Street<br />
Carnegie 180<br />
Baltimore, MD 21287<br />
(410) 955-6591<br />
Visit us on the web!<br />
www.hopkinspharmacy.org<br />
Group A no longer has a recommendation to use combination LABA + LAMA if monotherapy fails. 1,3<br />
There were no changes to treatment recommendations for patients categorized in Group B. In Group C,<br />
the initial recommendation is a LAMA, which differs from previous versions in which a combination<br />
LABA or LAMA with ICS was the initial recommendation. 1,3 This is because LABA + LAMA combination<br />
was proven superior to LABA + ICS in patients with a history of frequent exacerbations. 4 Similarly,<br />
patients classified as Group D previously were recommended to start with ICS + LABA, or ICS + LA-<br />
MA, with triple therapy as a next step. 1,3 The <strong>2017</strong> guidelines recommend combination LABA + LAMA<br />
first, with addition of ICS if symptoms persist. 1<br />
(continued on page 2)
Page 2 Volume 17; Issue 2<br />
A Summary of the <strong>2017</strong> GOLD Guidelines Update<br />
(continued from page 1)<br />
Addition of hospital discharge and follow-up criteria, including<br />
use of integrated care team<br />
The 2011-2015 versions of the GOLD guidelines provided criteria<br />
for discharge (e.g. ability to use inhaler properly, ability to walk<br />
across room without getting winded, etc.) and recommended follow-up<br />
within four to six weeks (e.g. reassessment of inhaler technique,<br />
status of comorbidities, etc.). 3 The updated guidelines shorten<br />
the recommended follow-up period to 4 weeks, citing a reduced<br />
rate of readmission in patients seen within the shorter timeframe.<br />
1,5 The guidelines also recommend additional follow-up<br />
three months post-discharge to ensure the patient has returned to<br />
stable clinical state. 1 While the guidelines do not mention the value<br />
of a pharmacist specifically, they do encourage the use of coordinated,<br />
integrated care for patients in all settings. Observational<br />
studies in patients with COPD have identified a relationship between<br />
poor inhaler use and symptom control. 6 The updated<br />
GOLD guidelines stress the importance of education and training<br />
in inhaler device technique, and assessment of dosing frequency<br />
and technique at each follow-up visit. 1<br />
References<br />
1. Global Strategy for the Diagnosis, Management and Prevention of<br />
COPD, Global Initiative for Chronic Obstructive Lung Disease<br />
(GOLD) <strong>2017</strong>. Available from http://goldcopd.org. Accessed December<br />
2016.<br />
2. Soriano JB, Lamprecht B, Ramirez AS, et al. Mortality prediction in<br />
chronic obstructive pulmonary disease comparing the GOLD 2007<br />
and 2011 staging systems: a pooled analysis of individual patient data.<br />
The Lancet Respiratory Medicine 2015; 3: 443-50.<br />
3. Global Strategy for the Diagnosis, Management and Prevention of<br />
COPD, Global Initiative for Chronic Obstructive Lung Disease<br />
(GOLD) 2016. Available from http://goldcopd.org. Accessed December<br />
2016.<br />
4. Wedzich JA, Banerji D, Chapman KR, et al. Indacaterolglycopyrronium<br />
versus salmeterol-fluticasone for COPD. N Engl J<br />
Med 2016; 374:222-34.<br />
5. Gavish R, Levy A, Dekel OK, et al The Association between hospital<br />
readmission and pulmonologist follow-up visits in patients with<br />
COPD. CHEST 2015; 148:375-81.<br />
6. Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains<br />
common in real life and is associated with reduced disease control.<br />
Respir Med 2011; 105:930-8.
Page 3 Volume 17; Issue 2<br />
Angiotensin-Converting Enzyme Inhibitor and Angiotensin Receptor Blocker:<br />
Cost Saving Projections via Therapeutic Interchange<br />
Meera Vachhani, PharmD, PGY1 Pharmacotherapy Resident<br />
The angiotensin-converting enzyme inhibitors (ACEI) and angiotensin<br />
receptor blockers (ARB) drug class review completed for<br />
the Johns Hopkins Health System reviewed efficacy, cost, and<br />
safety among these medications. Based on this information, the<br />
drugs’ formulary status and therapeutic interchange protocols were<br />
developed for the JHHS.<br />
The formulary agents selected within the ACEI and ARB drug<br />
classes are captopril, enalapril, enalaprilat, and lisinopril, and<br />
irbesartan, losartan, and valsartan, respectively. Therapeutic interchange<br />
protocols were developed to interchange nonformulary<br />
ACEIs to lisinopril, and nonformulary ARBs to losartan. The implementation<br />
of the therapeutic interchanges outlined above are<br />
projected to result in approximately $25,000 in cost savings annually.<br />
These projections are calculated based on 12-month volume<br />
history from McKesson. Of note, a new combination therapy,<br />
valsartan and sacubitril (Entresto®), was also added to the JHHS<br />
formulary with restriction to continuation of a home medication.<br />
New initiations are restricted to patients on a cardiology service or<br />
those with a cardiology consult.<br />
The ACEI/ARB therapeutic interchange protocols are currently<br />
being built within Epic. Communication to the department will be<br />
disseminated prior to the build changes going live in Epic. The<br />
therapeutic interchange details are outlined in Lexicomp.<br />
References:<br />
1. Furberg CD and Pitt B. Are all angiotensin-converting enzyme inhibitors<br />
interchangeable? J Am Coll Cardiol 2001; 37: 1456-60.<br />
2. Heran BS et al. Blood pressure lowering efficacy of angiotensin receptor<br />
blockers for primary hypertension. Cochrane Database of<br />
S y s t e m a t i c R e v i e w s 2 0 0 8 ; 4 : C D 0 0 3 8 2 2 . D O I :<br />
10.1002/14651858.CD003822.pub2.<br />
3. Matchar DB et al. Systematic review: comparative effectiveness of<br />
angiotensin-converting enzyme inhibitors and angiotensin II receptor<br />
blockers for treating essential hypertension. Ann Intern Med<br />
2008; 148: 16-29.<br />
Granisetron Transdermal System for the Prevention of<br />
Chemotherapy-Induced Nausea and Vomiting<br />
Olivia Akah, Notre Dame of Maryland University, PharmD Candidate 2019<br />
Lauren McBride, PharmD, BCOP, Clinical Pharmacy Specialist, Oncology<br />
Chemotherapy has helped millions of people in the fight against<br />
cancer by destroying cancerous cells. Unfortunately, healthy noncancerous<br />
cells are also destroyed in the process, leading to various<br />
unpleasant side effects. Chemotherapy-induced nausea and vomiting<br />
(CINV) is potentially the most severe and distressing adverse<br />
effect experienced by patients undergoing chemotherapy. To prevent<br />
CINV associated with chemotherapy regimens with a high<br />
emetic risk (i.e., incidence of emesis exceeds 90% if no antiemetics<br />
are administered), the American Society of Clinical Oncology<br />
(ASCO) currently recommends a 3-drug antiemetic regimen consisting<br />
of a NK-1 receptor antagonist (e.g., aprepitant, fosaprepitant)<br />
a type 3 serotonin (5-HT3) receptor antagonist (e.g., granisetron,<br />
ondansetron, palonosetron), and dexamethasone. 1,2<br />
Granisetron is a first generation selective inhibitor of type 3 serotonergic<br />
(5-HT3) receptors with little or no affinity for other serotonin<br />
receptors. It is an antiemetic indicated for the prevention of<br />
nausea and vomiting in patients receiving moderately and/or highly<br />
emetogenic chemotherapy regimens of up to 5 consecutive days<br />
of duration. The drug is structurally and pharmacologically related<br />
to ondansetron. The antiemetic activity of granisetron appears to<br />
be mediated both centrally and peripherally via inhibition of 5-<br />
HT3. 2 Serotonin receptors of the 5-HT3 type are located peripherally<br />
on vagal nerve terminals and centrally in the chemoreceptor<br />
trigger zone of the area postrema. During chemotherapy, mucosal<br />
enterochromaffin cells release serotonin, which stimulates 5-HT3<br />
receptors. This evokes vagal afferent discharge, inducing vomiting.<br />
3<br />
In select patients who are unable to swallow or digest tablets because<br />
of emesis, transdermal antiemetics such as granisetron transdermal<br />
system may be of value. In September 2008, the FDA approved<br />
the use of granisetron transdermal system (GTS) for<br />
CINV. The patch containing 3.1 mg of granisetron/24 hours is<br />
applied approximately 24 to 48 hours before the first dose of<br />
chemotherapy; maximum duration of the patch is 7 days. 3,4<br />
The efficacy and tolerability of transdermal granisetron for the<br />
control of CINV associated with moderately and highly emetogenic<br />
multi-day chemotherapy was assessed in a phase III clinical trial.<br />
The trial (n=641) was a randomized, non-inferiority, active control,<br />
double-blind, double-dummy, parallel group, multinational<br />
study that compared GTS to oral granisetron. Patients were randomized<br />
to oral (2 mg/day, 3-5 days) or transdermal granisetron<br />
(one GTS patch, 7 days), before receiving multi-day chemotherapy.<br />
The primary endpoint was complete control of CINV (i.e. no<br />
vomiting/retching, no more than mild nausea, no rescue medication)<br />
from chemotherapy initiation until 24 h after final administration.<br />
The pre-specified non-inferiority margin was 15%. Results<br />
showed that GTS displayed non-inferiority to oral granisetron;<br />
complete control was achieved by 60% of patients in the<br />
GTS group and 65% in the oral granisetron group (treatment difference,<br />
−5%; 95% confidence interval, −13% to +3%). Both<br />
treatments were well tolerated. 5<br />
The most common adverse effect of GTS is constipation. Skin<br />
reactions such as rash, redness, and itching may occur at the site of<br />
application. GTS is contraindicated in patients with known hypersensitivity<br />
to granisetron or to any of the components of the<br />
patch. The use of granisetron may mask a progressive ileus and/or<br />
gastric distention caused by the underlying condition. Development<br />
of serotonin syndrome has been reported with 5-HT3 receptor<br />
antagonists. Most reports have been associated with concomitant<br />
use of serotonergic drugs such as selective serotonin reuptake<br />
inhibitors (SSRI’s), mirtazapine, lithium, tramadol,<br />
(continued on page 4)
Page 4 Volume 17; Issue 2<br />
Granisetron Transdermal System for the Prevention of Chemotherapy-Induced<br />
Nausea and Vomiting<br />
(continued from page 3)<br />
etc. Safety and efficacy has not been established in pediatric patients.<br />
3,6<br />
Granisetron transdermal patch was recently added to the formulary<br />
at the Johns Hopkins Hospital with restrictions. It is restricted<br />
to oncology inpatients who have not responded to or are intolerant<br />
to at least 2 different agents (one of which must be a 5-HT3<br />
antagonist) with optimized dosing and frequency each for at least<br />
24 hours, or who respond to 5HT‐3 antagonists but are unable to<br />
transition from IV to PO antiemetics.<br />
References<br />
1. Basch E, Prestrud AA, Hesketh PJ, et al; American Society of Clinical<br />
Oncology. Antiemetics: American Society of Clinical Oncology<br />
clinical practice guideline update. J Clin Oncol 2011;29:4189-98.<br />
2. Granisetron [monograph]. In: LexiComp Online [online database].<br />
Hudson, OH: Lexi-Comp. Accessed December 2016.<br />
3. Food and Drug Administration. SANCUSO (Granisetron Transdermal<br />
System) prescribing information (September 2015). http://<br />
w w w . a c c e s s d a t a . f d a . g o v / d r u g s a t f d a _ d o c s /<br />
label/2015/022198s003lbl.pdf . Accessed December 2016.<br />
4. National Comprehensive Cancer Network (NCCN) Clinical Practice<br />
Guidelines in Oncology. Antiemesis (updated 2016). https://<br />
www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf<br />
Accessed December 2016.<br />
5. Boccia, R.V., Gordan, L.N., Clark, G. et al. Efficacy and tolerability<br />
of transdermal granisetron for the control of chemotherapy-induced<br />
nausea and vomiting associated with moderately and highly emetogenic<br />
multi-day chemotherapy: a randomized, double-blind, phase III<br />
study. Support Care Cancer 2011; 19:1609-17.<br />
6. Chabner B, A., Thompson E. C. Management of Adverse Effects of<br />
Cancer Therapy. Merck Manual Professional Version. https://<br />
www.merckmanuals.com/professional/hematology-and-oncology/<br />
principles-of-cancer-therapy/management-of-adverse-effects-ofcancer-therapy.<br />
Access January <strong>2017</strong>.<br />
NINJAs in the Children’s Center<br />
Bethany Sharpless, PharmD, PGY2 Pediatric Pharmacy Resident<br />
When one thinks of the word “ninja,” pediatric acute kidney injury<br />
(AKI) is generally not the first thing that comes to mind.<br />
“NINJA,” or Nephrotoxic Injury Negated by Just-in-time Action,<br />
is a multi-center quality improvement project with the goal of early<br />
identification of non-critically ill pediatric patients at risk for medication-induced<br />
AKI and reduction of the incidence and intensity<br />
of medication-induced AKI. The initiative, based out of Cincinnati<br />
Children’s Hospital Medical Center, began after a retrospective<br />
analysis of hospitalized children showed that rates of AKI increased<br />
from 16% to 45% in pediatric patients with exposure to ≥<br />
3 nephrotoxic medications. 1 In addition, pediatric evidence has<br />
shown that 5 or more days of aminoglycoside exposure was an<br />
independent risk factor for AKI, with rates of kidney injury increasing<br />
with days of therapy. 2 AKI is sometimes more easily<br />
missed in pediatric patients, as a quantitatively small change in<br />
serum creatinine (for example an increase from 0.3 to 0.6) represents<br />
an increase of two times baseline, which qualifies as AKI per<br />
the KDIGO (Kidney Disease Improving Global Outcomes) criteria.<br />
3 Johns Hopkins was the 13 th institution to join the NINJA<br />
collaborative and implemented the program in 2016.<br />
The criteria to identify patients who need closer renal monitoring<br />
are patients on ≥ 3 concomitant nephrotoxic medications or an<br />
aminoglycoside for ≥ 3 days. 4 The group intentionally excluded<br />
critically ill patients as those patients often have risk factors for<br />
nephrotoxicity beyond medications. In 2015, the Cincinnati project<br />
team reported 3.5 years of data. 5 During the period, decreases<br />
in AKI rate (2.96 to 1.06 AKI episodes per 1000 patient-days) as<br />
well as nephrotoxic medication exposure rate (11.64 to 7.24 exposures<br />
per 1000 patient-days) were observed. Multicenter collaborative<br />
data have not yet been published.<br />
While each institution has different workflows, the common goal<br />
is to decrease the intensity of AKI through increased awareness<br />
and monitoring. At JHH, Elys Bhatia of Clinical Analytics created<br />
Image source: Johns Hopkins Children Center<br />
(continued on page 5)
Page 5 Volume 17; Issue 2<br />
NINJAs in the Children’s Center<br />
(continued from page 4)<br />
Acyclovir<br />
Cidofovir<br />
NINJA 61 Medication List<br />
= Therapeutic Monitoring Recommended<br />
Gadopentetate<br />
dimeglumine<br />
(Magnevist)<br />
Ioxaglate meglumine<br />
and ioxaglate<br />
sodium<br />
Valacyclovir<br />
Ambisome Cisplatin Ganciclovir Ioxilan Pentamidine Valganciclovir<br />
Amikacin* Colistimethate Gentamicin* Ketorolac Piperacillin Valsartan<br />
Amphotericin B Cyclosporine* Ibuprofen Lisinopril<br />
Pamidronate disodium<br />
Piperacillin/Tazobactam<br />
Vancomycin*<br />
Aspirin Dapsone Ifosfamide Lithium* Sirolimus* Zoledronic acid<br />
Captopril<br />
Carboplatin<br />
Cefotaxime<br />
Diatrizoate meglumine<br />
Diatrizoate sodium<br />
Enalapril<br />
Indomethacin Losartan Sulfasalazine Zonisamide<br />
Iodixanol<br />
(Visipaque)<br />
Iohexol<br />
(Omnipaque)<br />
Mesalamine<br />
Methotrexate*<br />
Ceftazidime Enalaprilat Iopamidol (Isovue) Mitomycin<br />
Tacrolimus*<br />
Tenofovir<br />
Ticarcillin/<br />
Clavulanic Acid<br />
Cefuroxime Foscarnet Iopromide Nafcillin Tobramycin*<br />
Celecoxib<br />
Gadoextate disodium<br />
(Eovist)<br />
Ioversol Naproxen Topiramate<br />
a Qlikview dashboard that provides the rest of the NINJA team<br />
(Jeff Fadrowski, MD, Elizabeth Goswami, PharmD, and Emma<br />
Sexton, RN) a daily report of patients exposed to nephrotoxic<br />
medications, as well as patients meeting criteria for AKI. If a patient<br />
meets the criteria for increased risk of AKI, the NINJA<br />
team visits the patient’s primary team on the floor with a<br />
“NINJA Alert”. During the conversation, the NINJA team recommends<br />
daily serum creatinine monitoring as well as minimization<br />
of nephrotoxic medications as clinically appropriate. The<br />
NINJAs make the team aware of the patient’s baseline serum<br />
creatinine and tell the team if the patient has AKI. Patients are<br />
followed for the duration of nephrotoxic medication exposure,<br />
and patients with AKI are followed until AKI has resolved for 48<br />
hours.<br />
Although this project currently impacts only our non-critically ill<br />
pediatric patients, awareness of medication-associated AKI risk is<br />
important regardless of patient age. Acute kidney injury is a cause<br />
of patient morbidity and has been shown to have long-term implications<br />
on a patient’s renal function. 6 Pharmacists have an opportunity<br />
to promote early detection of patients with medication<br />
risk factors for AKI, and early intervention by pharmacists can<br />
result in reduction in nephrotoxic medication exposure as well as<br />
incidence of AKI.<br />
References:<br />
1. Moffett B, Goldstein S. Acute kidney injury and increasing nephrotoxic-medication<br />
exposure in noncritically-ill children. Clinical<br />
Journal of the American Society of Nephrology. 2011;6:856-63.<br />
2. Zappitelli M, Moffett B, Hyder A, Goldstein S. Acute kidney injury<br />
in non-critically ill children treated with aminoglycoside antibiotics<br />
in a tertiary healthcare centre: a retrospective cohort study. Nephrology<br />
Dialysis Transplantation. 2010;26:144-50.<br />
3. Summary of Recommendation Statements, KDIGO Clinical Practice<br />
Guideline for Acute Kidney Injury. Kidney International Supplements.<br />
2012;2:8-12.<br />
4. Goldstein S, Kirkendall E, Nguyen H et al. Electronic health record<br />
identification of nephrotoxin exposure and associated acute kidney<br />
injury. Pediatrics. 2013;132:e756-67.<br />
5. Goldstein S, Mottes T, Simpson K et al. A sustained quality improvement<br />
program reduces nephrotoxic medication-associated<br />
acute kidney injury. Kidney International. 2016;9:212-21.<br />
6. Menon S, Kirkendall E, Nguyen H, Goldstein S. Acute kidney injury<br />
associated with high nephrotoxic medication exposure leads to<br />
chronic kidney disease after 6 Months. The Journal of Pediatrics.<br />
2014;165:522-7.
Page 6 Volume 17; Issue 2<br />
A Centralized Electronic Health Record: How Close Are We?<br />
Ben Iredell, PharmD, PGY1 Medication Systems and Operations Resident<br />
A patient is admitted with pneumonia on Friday evening, but she<br />
claims she has a history of anaphylaxis with multiple antibiotics.<br />
She does not recall which ones specifically, and her out of state<br />
Primary Care office will not be open until Monday. Skin testing<br />
aside, the patient may have to go the weekend without antibiotics.<br />
These situations unfortunately happen frequently due to a lack of<br />
universal integration among health systems’ Electronic Health<br />
Records (EHR). However, there are efforts underway to connect<br />
health systems across the country.<br />
In 2004, President George W. Bush set a goal that by 2014, most<br />
Americans should have access to secure electronic health records. 3<br />
The executive order foresaw that medical information would follow<br />
consumers from provider to provider and that each clinician<br />
would have a patient’s complete medical record. Now, in <strong>2017</strong>, we<br />
still have yet to reach that goal. A government initiative, endingthedocumentgame.gov,<br />
was implemented to reduce the number<br />
of duplicate documents and tests from provider to provider. 3 In<br />
2009, Congress passed the Health Information Technology for<br />
Economic and Clinical Health Act (HITECH Act), which authorized<br />
the Department of Health and Human Services to spend $30<br />
billion on the expansion of Heath Information Technology<br />
(HIT). 3 The act defines “meaningful use” in terms of HIT and<br />
improving clinical outcomes using EHR technology. 2<br />
While progress at the national level lags, some states are having<br />
success with creating regional Health Information Exchanges<br />
(HIE). The California Integrated Data Exchange (Cal INDEX) is<br />
a nonprofit organization that is funded by two large health insurance<br />
companies in the state. The organization maintains a centralized<br />
EHR that is populated with patient health information by<br />
providers and by insurance claims. While not completely comprehensive,<br />
this is a good start to a one-stop health record. Other<br />
states have created or have plans for similar systems, including<br />
Georgia, Arkansas, and Virginia.<br />
In Maryland, the Chesapeake Regional Information System for our<br />
Patients (CRISP) organization is leading the way towards a centralized<br />
EHR in Maryland and the District of Columbia. 1 The CRISP<br />
Clinical Query Portal is a free tool available now to ambulatory<br />
practices, and the Prescription Drug Monitoring Program<br />
(PDMP), which allows for a common repository of data related to<br />
controlled substance prescribing and dispensing, is one of the<br />
more mature aspects of Maryland’s program.<br />
Besides clinical benefits such as enabling faster diagnoses and improving<br />
comprehensive care, a centralized EHR will save health<br />
systems money. For example, a complete patient record will show<br />
the provider all tests performed on the patient, which may eliminate<br />
duplicate or unnecessary tests. Additionally, providers and<br />
health systems will not spend as much time learning new systems<br />
or transferring health information if it is already synced across all<br />
health systems.<br />
While 2014 has come and gone and nation-wide connected patient<br />
records has yet to be achieved, progress is being made on the local<br />
and national levels. CRISP, while a relatively new system, has the<br />
potential to connect patient records for hospitals and health systems<br />
in Maryland and the District of Columbia. With time, the<br />
hope is that every health system in America will be connected and<br />
each patient’s antibiotic allergies (and other health-related information)<br />
will be available to all providers.<br />
References:<br />
1. CRISP Clinical Query Portal. CRISP. https://www.crisphealth.org/<br />
services/crisp-clinical-query-portal/. Accessed December 2016.<br />
2. Health Information Technology. American College of Emergency Physicians.<br />
https://www.acep.org/Advocacy/Health-Information-<br />
Technology/. Accessed December 2016.<br />
3. Kendall, D., Quill, E. A lifetime electronic health record for every<br />
american. Third Way: Fresh Thinking. http://www.thirdway.org/<br />
report/a-lifetime-electronic-health-record-for-every-american. Accessed<br />
December 2016.<br />
4. Koppel, R.. What do we know about medical errors associated with<br />
electronic medical records? The Health Care Blog. http://<br />
thehealthcareblog.com/blog/2016/01/11/what-do-we-know-aboutmedical-errors-associated-with-electronic-medical-records/.<br />
cessed December 2016.<br />
Ac-<br />
Our “Epic” Summer<br />
Detron Brown, PharmD Candidate, Howard University College of Pharmacy, Class of 2018<br />
Megan Bereda, PharmD Candidate, Purdue College of Pharmacy, Class of 2018<br />
On July 1 st of 2016, the Johns Hopkins Hospital finalized its transition<br />
to Epic as its exclusive electronic health record. This was<br />
one of the largest changes in Hopkins’ history and meant extensive<br />
workflow and standard operating procedure changes. Many were<br />
left feeling uneasy of being able to utilize the system while still<br />
providing the highest level of care. As summer interns in the Critical<br />
Care and Surgery (CCS) Pharmacy, we were tasked with easing<br />
the stress of the transition.<br />
While undergoing the Epic-provided training alongside our<br />
technicians, we recognized that while the training was a great introduction<br />
to the software, we could further serve our technicians<br />
by providing hands-on use of the software. We recognized that if<br />
the training included more real-life experiences and scenarios, the<br />
technician level of comfort would increase significantly. Together<br />
we compiled a technician training program that focused on the six<br />
major functions that we felt were the most vital for technician<br />
success in the new Epic system.<br />
Eighty-six percent of the full-time CCS technicians were cotrained<br />
prior to “Go-Live” on July 1, 2016. Through our individual<br />
training sessions, we were able to teach each of the major func-<br />
(continued on page 7)
Page 7 Volume 17; Issue 2<br />
Our “Epic” Summer<br />
(continued from page 6)<br />
tions and observe each technician’s competence with them. Upon<br />
completion of the mock scenarios prepared in the Epic Hyperspace<br />
training modules, we were able to directly serve as a resource<br />
and observe each technician’s “real-time” competence.<br />
Many technicians cited this training module as a significant factor<br />
in increasing their comfort in maneuvering through the new system.<br />
At ASHP’s Midyear Clinical Meeting in December, we presented<br />
our work and described the benefit of site specific one-onone<br />
training versus non-specific group training. We are forever<br />
grateful for experiences we were able to have over the summer<br />
and the support we received from the CCS pharmacy staff and<br />
summer internship program.<br />
The Johns Hopkins Hospital at Fundación Santa Fe de Bogotá<br />
Mustafa Sidik, CPhT<br />
The Department of Pharmacy had the opportunity to send a pharmacy<br />
technician to the Fundación Santa Fe de Bogotá, an international<br />
affiliate of Johns Hopkins, to present on pharmacy technician<br />
practice during a two day national pharmacy conference. The<br />
hospital had no formal pharmacy technician role, and was seeking<br />
to learn how to effectively implement a successful, sustainable<br />
pharmacy technician position. I was fortunate to be chosen as the<br />
Johns Hopkins representative and wanted to share my experience.<br />
I presented two lecture to approximately 300 pharmacy professionals<br />
from across Colombia: one on the role of the pharmacy<br />
technician in the United States and the other on the role of the<br />
pharmacy technician in medication safety. Following the lectures I<br />
discussed specific pharmacy technician roles with pharmacy and<br />
hospital executives. A tour of the hospital, including the central<br />
pharmacy and many of its satellites rounded out the first day. The<br />
second day was spent in targeted focus groups, where discussions<br />
were held with the hospital’s current pharmacists, associates, and<br />
administrators, as well as representatives from the country’s Board<br />
of Pharmacy and Health Ministry. After the two days, the consensus<br />
was reached that while it is critical for the role of the pharmacy<br />
technician to develop, it is equally as critical for the hospital’s<br />
program to be rooted firmly in education and training to adequately<br />
prepare individuals to be competent pharmacy technicians.<br />
Overall, this trip was an eye-opening experience to all that we take<br />
for granted in pharmacy practice, not only at The Johns Hopkins<br />
Hospital, but the United States as well. Technologies such as Pyxis,<br />
Epic, and DoseEdge which we rely on heavily, are not accessible<br />
in many other countries. This opportunity gave me a newfound<br />
appreciation for the level of progress that The Johns Hopkins<br />
Hospital Department of Pharmacy has achieved and the steps<br />
we are taking to advance pharmacy practice.<br />
Indispensable Impact<br />
Kristen Holt, PharmD, MPH, Pharmacy Administration<br />
Last year, clinical pharmacists made over 90,000 recommendations that benefited the care of patients at The Johns Hopkins Hospital.<br />
These interventions were incorporated by the care team 98% of the time. Using your imagination; how could this patient’s story have<br />
unfolded differently if this pharmacist was not there to intervene?<br />
A pregnant patient was ordered haloperidol short acting 100 mg intramuscularly (IM) once. The pharmacist paged the provider to inform<br />
her that haloperidol should be avoided in the first trimester of pregnancy since there is the potential for limb malformations. In addition,<br />
100 mg IM once should be the long acting and not the short acting formulation. The drug was discontinued.<br />
Pharmacists at Johns Hopkins are dedicated to help patients benefit from their medications safely, effectively, and affordably. Each<br />
scenario illustrates a clinical pharmacist’s indispensable impact.
Page 8 Volume 17; Issue 2<br />
Departmental Continuing<br />
Education<br />
“Medications Associated with<br />
Exacerbation of Heart<br />
Failure”<br />
ACPE Accredited CE<br />
Presentation<br />
Laura Fuller, PharmD, BCPS<br />
Dr. Ken Shermock appointed to the 2018 Pharmacy Forecast<br />
Advisory Committee...Congratulations!<br />
Dr. Kenneth Shermock, PharmD, PhD was invited to be a member<br />
of the ASHP Foundation’s 2018 Pharmacy Forecast Advisory<br />
Committee. The Foundation is celebrating the publication of the<br />
Pharmacy Forecast <strong>2017</strong> in the January 15th edition of AJHP and<br />
is moving forward quickly with planning for the 2018 edition.<br />
<strong>February</strong> 2, <strong>2017</strong><br />
3:00 PM - 4:00 PM, AIP<br />
Conference Room<br />
<strong>February</strong> 6, <strong>2017</strong><br />
12:00 PM - 1:00 PM, Zayed 2117<br />
(Lunch provided)<br />
<strong>February</strong> 15, <strong>2017</strong><br />
7:00 AM - 8:00 AM, AIP<br />
Conference Room<br />
The Pharmacy Forecast Report is a high-profile and impactful<br />
project of the ASHP Foundation. The Report predicts important<br />
developments in eight domains that are likely to challenge pharmacy<br />
practice leaders in hospitals and health systems. Its purpose<br />
is to improve the effectiveness of leaders in hospital and healthsystem<br />
pharmacy practice by helping them plan for the future.<br />
Pharmacy Forecast reports the results of a survey of trend watchers in health-system pharmacy,<br />
analyzes predicted trends, and presents more than 40 authoritative, actionable strategic recommendations<br />
to pharmacy practice leaders. As a member of the committee, Dr. Shermock will<br />
participate in the identification of key issues and trends in healthcare.<br />
<strong>February</strong> Birthdays<br />
Sun Mon Tue Wed Thu Fri Sat<br />
“Herbal and Homeopathic<br />
Medications:<br />
From Early Civilizations to<br />
Modern Day”<br />
ACPE Accredited CE<br />
Presentation<br />
Robert Green, PharmD<br />
<strong>February</strong> 21, <strong>2017</strong><br />
12:00 PM - 1:00 PM, Hurd Hall<br />
(Lunch provided)<br />
5 6<br />
Laura Hatfield<br />
7<br />
Dinesh Pun<br />
Annette<br />
Rowden<br />
Julie<br />
Waldfogel<br />
1<br />
Maher Ebeid<br />
Ubong Ibok<br />
Maika Patino<br />
8<br />
Daron Fleet<br />
Laura Fuller<br />
2<br />
Marie Lymon<br />
Victoria<br />
Sammarco<br />
Regina Yun<br />
9<br />
Jason<br />
Topolski<br />
3<br />
Kumaran<br />
Ramakrishnan<br />
Tyrell Schaffer<br />
Hunter Smoot<br />
Cathy Walker<br />
10<br />
Sharaun<br />
Harvell<br />
4<br />
Jason Choe<br />
Ahmed Eid<br />
Eric Fourhman<br />
11<br />
Michelle McCoy<br />
<strong>February</strong> 28, <strong>2017</strong><br />
3:00 PM - 4:00 PM, AIP<br />
Conference Room<br />
12<br />
Manisha Hong<br />
Adrienne Taylor<br />
Javier Vazquez<br />
13<br />
Carrie Moon<br />
14<br />
Kaitlyn Borries<br />
15<br />
Sharon Addo<br />
Christine<br />
Collins<br />
16<br />
Amy Shofner<br />
17<br />
Sharon<br />
Johnson<br />
18<br />
Michael Evans<br />
Sean Lasota<br />
Modestus<br />
Nwadike<br />
Pharmacotherapy Rounds will<br />
not be held in <strong>February</strong> due to<br />
residency interviews.<br />
19<br />
Rachel Zamora<br />
20<br />
Julie Gibbons<br />
21<br />
Kristin Gilmore<br />
22<br />
Leah<br />
Georgandellis<br />
23<br />
Rachel Lim<br />
24<br />
Dennisse<br />
Rubio Colon<br />
Stanley Okeke<br />
25<br />
Wayne Borkoski<br />
Ricky Haskey<br />
26<br />
Elizabeth<br />
Goswami<br />
Traize Takla<br />
Deanah Thomas<br />
Tricie Williamson<br />
27 28<br />
Nicole Arwood<br />
Michael<br />
Goldenhorn<br />
Jennifer<br />
Redmon<br />
Michael Veltri