Semester 1 Notebook-Martin
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Learning Goal for this section:<br />
Explain and copare nuclear reactions, radioactive decay, fission, and the<br />
energy, changes associated with them and their associated safety issues.<br />
Notes Section:<br />
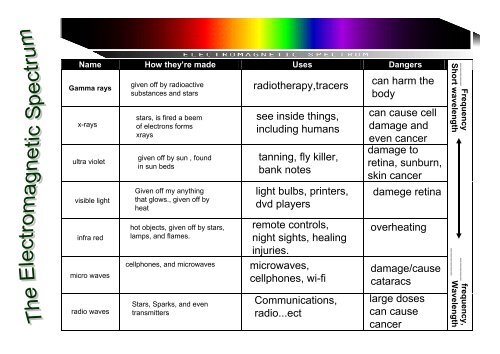
Nucleus<br />
N-1amu-none<br />
P-1amu-positve<br />
E-none-negative<br />
carbon 14 could be radioactive because it<br />
had a radioactive isotope.<br />
carbon makes up everything, things have<br />
specific amounts of carbon 14.<br />
Beta particles have the mass of an electron, theres positive and negative beta<br />
particles. Neutrons are both positive and negative, you take away the neutron<br />
it becomes positive<br />
Positive beta-Positron, has<br />
practically no charge.<br />
Can take away positive<br />
charge from neutron and<br />
make a negative, vise<br />
versa.<br />
If you do that is goes down by one EX: poloniom<br />
would become bismouth.<br />
Gamma(small amount)- ENRGY. -Not a huge amount but can be<br />
dangerous<br />
Half life-loses protons, loses power, becomes weaker.<br />
Iodine131 20mg has radioactivity- half life 8 days